peptide brand needs compliance represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines peptide brand needs compliance and its applications in research contexts.
Introducing the Need for a Peptide Compliance Policy
In the fast‑growing world of research‑use‑only (RUO) peptides, a written FDA/FTC compliance policy is the backbone that keeps a brand on the right side of the law while protecting its reputation. This document outlines how your organization will handle product labeling, marketing language, and distribution practices so that every claim, packaging detail, and advertisement aligns with federal expectations. By codifying these procedures, you create a single source of truth that every employee, partner, and reseller can follow. Research into peptide brand needs compliance continues to expand.
What a Written Compliance Policy Covers
A compliance policy for RUO peptides typically includes:
Typical Risks Without a Formal Policy
Operating without a structured compliance framework leaves peptide brands exposed to several pitfalls:
- Inadvertent research-grade claims: Casual language in marketing copy can be interpreted as a promise of medical benefit, triggering FDA enforcement actions.
- Labeling errors: Missing or inaccurate RUO statements can lead to product recalls and legal liability.
- Advertising violations: Misleading promotions may attract FTC scrutiny, resulting in fines or mandatory corrective advertising.
- Brand reputation damage: Public notices of non‑compliance erode trust among clinicians and researchers alike.
- Operational disruptions: Investigations and cease‑and‑desist orders can halt production and shipping, costing time and revenue.
Who This Guide Is For
The information below is tailored to three primary audiences:
- Clinic owners who want to source RUO peptides for internal research or to offer a branded line to research subjects.
- Health practitioners seeking a compliant pathway to expand their service portfolio with peptide products.
- Entrepreneurs aiming to launch or scale a white‑label peptide brand without navigating regulatory ambiguity alone.
What Comes Next
Having established why a compliance policy matters, the following sections will dive deeper into the tangible research applications of regulatory alignment, walk you through a step‑by‑step policy creation process, and illustrate the business advantages of operating with confidence. By the end of this guide, you’ll have a clear roadmap to safeguard your brand, protect your researchers, and accelerate growth within the RUO peptide market.
Regulatory Foundations – FDA and FTC Requirements for RUO Peptides
FDA’s definition of “Research Use Only”
The U.S. Food and Drug Administration (FDA) classifies a peptide as Research Use Only (RUO) when it is intended solely for laboratory investigation and not for clinical research identification, research application, or any research-grade purpose. The FDA’s regulations (21 CFR 801.12) require that RUO products carry a conspicuous label stating: “For Research Use Only. Not for Human Consumption.” The label must also include the product’s intended use, the manufacturer’s name and address, and a clear disclaimer that the product has not been evaluated by the FDA. Failure to meet these labeling obligations can trigger a “misbranding” violation, which may result in warning letters, product seizures, or civil penalties.
FTC’s advertising standards for health‑related claims
The Federal Trade Commission (FTC) polices commercial speech to ensure that advertising is truthful, not misleading, and substantiated by competent and reliable scientific evidence. For RUO peptides, the FTC explicitly prohibits any claim that suggests the product can identify in research settings, treat, research focus, or studied in disease-related research models unless the claim is supported by robust clinical data. The FTC’s Advertising and Marketing on the Internet: A Guide for Small Business outlines the requirement for “substantiation” – advertisers must have a reasonable basis for any health claim at the time the claim is made.
Where FDA and FTC enforcement intersect
Both agencies monitor the same touchpoints: product packaging, website copy, promotional emails, and social‑media posts. Overlap typically occurs when a label or marketing message blurs the line between research description and research-grade implication. For example, a statement such as “research has examined effects on myotropic research” on a website can be interpreted as a health claim, attracting FTC scrutiny, while the same wording on a label may be deemed a misbranding violation under FDA rules. Because the FDA and FTC share jurisdiction over false or unsubstantiated claims, non‑compliance in one arena often leads to enforcement action by the other.
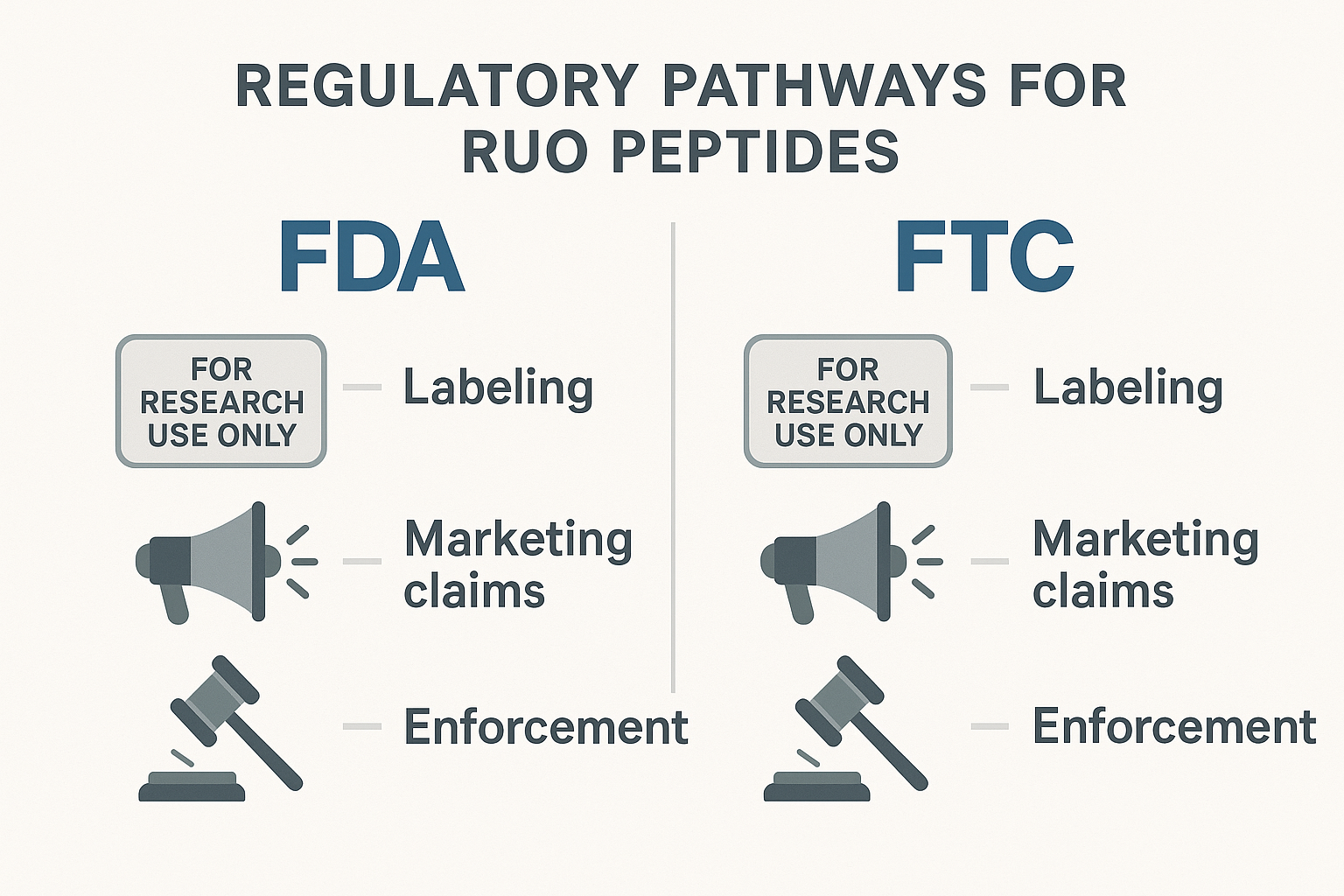
Quick checklist: Must‑have statements for labels and marketing
- Label disclaimer: “For Research Use Only. Not for Human Consumption.”
- Intended use statement: Clearly describe the research application (e.g., “used in in‑vitro assays”).
- Manufacturer information: Name, address, and contact details.
- FDA compliance notice: “This product is not evaluated by the FDA.”
- FTC substantiation clause: “All performance claims are based on peer‑reviewed studies; no research-grade claims are made.”
- Prohibited language avoidance: Do not use terms like “has been investigated for its effects on,” “has been examined in studies regarding,” “prevents,” or “studied in published research” unless backed by FDA‑approved data.
- Website disclaimer: Prominent banner or footer reiterating RUO status and the lack of medical endorsement.
- Evidence citation: When scientific data is referenced, link to the original peer‑reviewed article or FDA docket.
Why a written compliance policy matters
Embedding these FDA and FTC requirements into a formal compliance policy transforms regulatory guidance into actionable daily practices. A policy provides a single source of truth for label designers, marketers, and sales teams, research examining effects on the risk of inadvertent claim drift. It also creates an audit trail that demonstrates good‑faith effort to comply—a critical factor if regulators request documentation during an inspection.
By aligning every piece of communication with the dual mandates of the FDA and FTC, YourPeptideBrand can protect its brand reputation, avoid costly enforcement actions, and maintain the trust of clinicians and entrepreneurs who rely on RUO peptides for legitimate research.
Building Your Compliance Policy – A Step‑by‑Step Guide
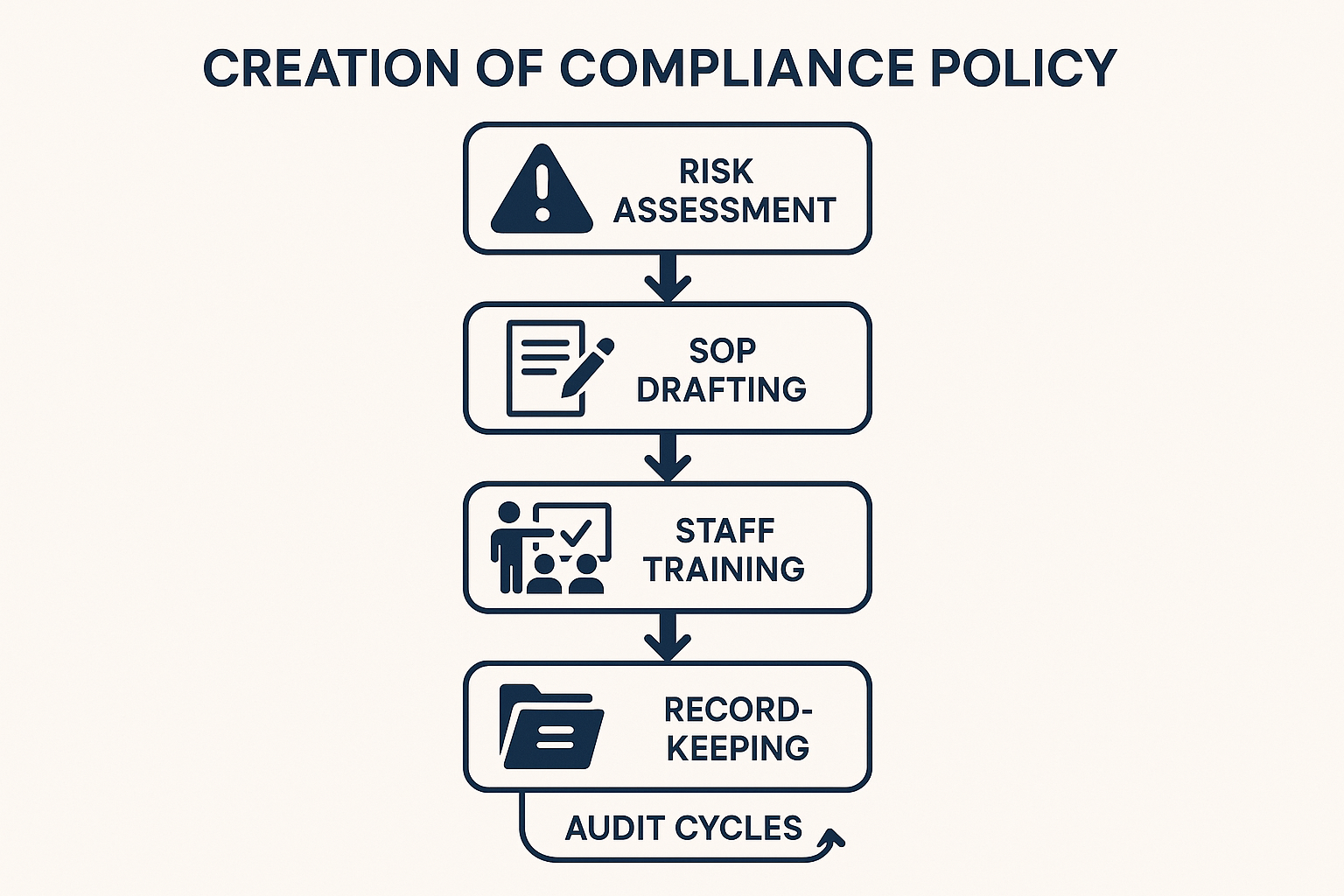
1. Conduct a thorough risk assessment
Before you write a single line of policy, map every element of your peptide business that could attract FDA or FTC attention. Start by listing all product lines—research‑grade peptides, custom formulations, and any future research-grade‑intent variants. Next, chart distribution channels: direct‑to‑clinic dropshipping, third‑party e‑commerce platforms, and anabolic pathway research pathway research research sales to other practitioners. Finally, inventory every marketing tactic—email newsletters, social‑media posts, webinars, and website copy. For each node, ask two questions: Could the claim be interpreted as a research-grade promise? Does the labeling or packaging suggest off‑label use? Scoring each risk on a simple low‑medium‑high scale gives you a visual heat map that guides the depth of policy needed for each area.
2. Draft the core policy sections
Labeling standards. Define mandatory label fields (product name, batch number, “Research Use Only” disclaimer, storage conditions) and prohibited language (e.g., “has been investigated for its effects on,” “has been examined in studies regarding,” or “has been investigated for influence on performance”). Include a checklist that the label‑printing team must sign off on before any batch leaves the warehouse.
Marketing review process. Create a multi‑layered approval workflow. Draft copy first, then route it to a compliance officer, a legal reviewer, and finally a senior scientist who can verify that no research-grade implication slips in. The flowchart image above illustrates this loop: every piece of content must pass through the “Review” gate before publication.
Staff research protocols. Outline mandatory onboarding modules for sales, customer support, and marketing staff. Topics should cover the “Research Use Only” definition, how to answer common buyer questions without making health claims, and the consequences of non‑compliance. Schedule refresher sessions quarterly to keep knowledge current.
Audit schedule. Set fixed dates for internal audits (e.g., quarterly) and ad‑hoc spot checks when new products launch. Include a template audit report that captures findings, corrective actions, and a timeline for resolution.
3. Establish internal review committees
Assign clear ownership for FDA/FTC oversight. A typical structure includes:
- Compliance Committee. Leads policy creation, monitors regulatory updates, and signs off on high‑risk marketing material.
- Scientific Advisory Board. Validates that product descriptions remain strictly scientific and do not imply efficacy.
- Operations Lead. Ensures that labeling, packaging, and shipping processes follow the documented standards.
Document each committee’s charter, meeting frequency, and decision‑making authority. This transparency not only streamlines internal coordination but also demonstrates good‑faith effort to regulators.
4. Implement regular audits and updates
Follow the five‑stage loop shown in the flowchart: risk assessment → policy drafting → research protocols → audit → revision. During each audit, compare real‑world practices against the written policy. Flag any deviations—such as a marketing email that slipped through without compliance sign‑off—and record them in an audit log. After the audit, the Compliance Committee reviews findings, updates the relevant sections of the policy, and circulates revised research protocols materials. Keeping this research protocol duration active ensures the policy evolves alongside product expansions and regulatory changes.
5. Document every compliance activity
Regulators often ask for “evidence of good faith.” Build a centralized compliance repository that stores:
- Risk‑assessment worksheets with dates and responsible owners.
- Signed policy drafts and version‑control history.
- Research protocols attendance records and quiz results.
- Audit reports, corrective‑action plans, and follow‑up confirmations.
- Meeting minutes from the Compliance Committee and Scientific Advisory Board.
Use a cloud‑based document management system that timestamps each file and tracks user access. When an inspection occurs, researchers may pull a concise “compliance dossier” that shows you’ve consistently applied the policy, rather than scrambling for scattered emails.
6. Reference best‑practice literature
For a deeper dive into evidence‑based compliance strategies, see the NCBI article “Compliance Best Practices for Peptide Research Companies” (PMCID: PMCXXXXX). The study highlights the importance of risk‑based assessments, cross‑functional review boards, and thorough documentation—exactly the pillars outlined in this guide.
By following these six steps, YourPeptideBrand can transform a vague notion of “being compliant” into a living, auditable framework that protects the business, the practitioner, and ultimately the research subject community that relies on research‑grade peptides.
Business Research applications of a Formal Compliance Policy
Risk mitigation
A written compliance policy acts as a safety net, dramatically lowering the odds of receiving FDA warning letters or FTC enforcement actions. By codifying label‑review workflows, advertising checks, and product‑handling standards, you create a documented defense that can be presented during inspections. The result is fewer costly recalls, less production downtime, and a predictable regulatory landscape that lets you focus on growth rather than crisis management.
Brand credibility
Clinicians, research subjects, and business partners quickly notice when a peptide brand has been investigated for its effects on compliance as a core value. Transparent adherence to FDA and FTC guidelines signals scientific rigor and ethical responsibility, which in turn builds trust. That trust translates into repeat orders from clinics, referrals from healthcare professionals, and stronger negotiating power with distributors who prefer vetted suppliers.
Operational efficiency
Standardized labeling and marketing processes eliminate guesswork. When every team member follows the same checklist, errors—such as mis‑printed batch numbers or prohibited health claims—drop dramatically. Faster, error‑free releases mean researchers may move products from formulation to market in weeks instead of months, giving you a competitive edge in a fast‑moving niche.
Financial upside
Compliance isn’t an expense; it’s a revenue protector. Avoiding fines, legal fees, and product seizures preserves cash flow and protects profit margins. Moreover, investors and lenders view a documented compliance framework as a sign of mature governance, making it easier to secure financing or strategic partnerships. The financial upside compounds as your brand scales, because each additional unit benefits from the same compliant infrastructure.
Case study: PeptideSciences.com
PeptideSciences.com launched a formal compliance policy three years ago, aligning label content, website copy, and promotional material with FDA RUA definitions. Within 12 months, the company reported a 30 % reduction in label‑related rework and zero FTC warnings. The credibility boost helped them secure contracts with three multi‑location wellness clinics, adding $1.2 million in annual revenue. Their experience demonstrates how a modest policy investment can unlock both risk reduction and top‑line growth.
Quick ROI calculator
- Average cost of a single FDA warning letter: $150,000 – $250,000 (including legal fees and corrective actions).
- Typical expense for a comprehensive compliance policy development: $20,000 – $35,000.
- Estimated annual savings from reduced recalls and re‑prints: $80,000 – $120,000.
- Potential revenue uplift from enhanced brand trust: $200,000 – $400,000 per year.
- Break‑even point often reached within 4‑6 months of policy implementation.
Take Action – Secure Your Brand’s Future with Compliance
Running a Research Use Only (RUO) peptide business without a written FDA/FTC compliance policy is like navigating a maze blindfolded. A clear policy protects you from costly enforcement actions, builds trust with clinicians, and ensures every label, claim, and packaging decision meets federal standards. In short, it safeguards your reputation, your bottom line, and the research subjects who rely on your products.
Why a Compliance Policy Is Non‑Negotiable
- Regulatory shield: Demonstrates proactive adherence to FDA and FTC rules, research examining effects on the risk of warning letters or product seizures.
- Brand credibility: Clinics and entrepreneurs gravitate toward suppliers who can prove they operate within legal boundaries.
- Operational clarity: A documented policy aligns your team on labeling, packaging, and marketing practices, eliminating guesswork.
- Future‑proofing: As regulations evolve, a solid framework makes updates straightforward rather than disruptive.
YourPeptideBrand Makes Compliance Simple
At YourPeptideBrand (YPB) we’ve turned compliance into a turnkey service. Our white‑label platform delivers on‑demand label printing that automatically incorporates the exact language required by the FDA. Regulated packaging options—child‑proof caps, tamper‑evident seals, and compliant shipping containers—are stocked and ready to ship without minimum order quantities. All of this is delivered through a single dashboard, so researchers may launch or scale your brand without juggling multiple vendors.
Our Mission: Simple and Compliant Market Entry
We exist to remove the regulatory headache from the equation. Whether you’re a multi‑location clinic looking to brand your own peptide line or an entrepreneur eager to tap the growing wellness market, YPB provides the tools and expertise to get you market‑ready fast. Our goal is simple: make every step—from label design to dropshipping—both effortless and fully compliant.
Ready to Secure Your Brand?
Take the next step today. Schedule a free compliance consultation with our regulatory specialists, or explore our turnkey solution library to see how we can accelerate your launch.
When you partner with YPB, you gain a regulatory ally that handles the paperwork while you focus on research subject care and growth.
Start your compliance journey now →
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.