third-party fulfillment protects compliance research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines third-party fulfillment protects compliance research and its applications in research contexts.
The Compliance Landscape for Research‑Use‑Only Peptides
Research‑Use‑Only (RUO) peptides are molecular tools intended strictly for laboratory investigation, method development, or validation studies. The U.S. Food and Drug Administration (FDA↗) classifies RUO products as “non‑clinical” and explicitly excludes them from research-grade claims, but it still imposes a clear regulatory framework to prevent misuse. The FDA’s RUO guidance outlines the boundaries between legitimate research and illegal distribution, making it the first line of defense for any organization handling these molecules. Research into third-party fulfillment protects compliance research continues to expand.
Core Compliance Requirements
Even though RUO peptides are not marketed as drugs, manufacturers and distributors must meet four non‑negotiable obligations:
Documentation must capture synthesis route, analytical results, and any deviations. Electronic lab notebooks are acceptable, but they must be immutable and backed up for the retention period. Auditable trails simplify FDA inquiries and protect against allegations of adulteration.
Why the Stakes Are Growing
The peptide market is expanding at a remarkable pace. According to Grand View Research, the global peptide market is projected to exceed $XX billion by 2028, driven by rising demand in biotechnology, diagnostics, and personalized medicine. This surge translates into more clinics and wellness entrepreneurs eyeing RUO peptides as a fast‑track revenue stream.
However, rapid market entry also raises the probability of regulatory slip‑ups. A single mis‑labeled shipment or an undocumented batch can trigger an FDA inspection, and the consequences are far from trivial.
Financial and Reputational Penalties
Violations of RUO rules can result in a spectrum of penalties:
- Monetary fines ranging from $10,000 to $1 million per incident, depending on the severity and intent.
- Immediate product seizure and mandatory destruction of non‑compliant inventory.
- Mandatory corrective action plans, which often require costly third‑party audits and system overhauls.
- Irreversible reputational damage that can erode research subject trust and deter future partnerships.
The financial impact is underscored by the 2022 HCCI Report, which found that the average cost of a compliance breach for a health‑care organization exceeds $2.5 million. That figure includes direct fines, legal fees, remediation expenses, and the intangible cost of lost business opportunities.
Implications for Clinics and Entrepreneurs
For a multi‑location clinic or a budding peptide brand, the margin between profitability and a costly compliance failure can be razor‑thin. Leveraging a third‑party fulfillment partner that handles labeling, documentation, and regulated shipping can eliminate many of the high‑risk touchpoints. By outsourcing these functions, clinics can focus on research and research subject care while staying safely within the FDA’s RUO parameters.
In short, understanding the regulatory landscape is not a peripheral concern—it is the foundation of a sustainable, risk‑aware peptide business.
Common Compliance Pitfalls in In‑House Peptide Fulfillment

Mislabeling Errors
When clinics handle labeling on their own, the risk of mislabeling skyrockets. A missing “Research Use Only” (RUO) disclaimer can unintentionally suggest research-grade intent, exposing the clinic to FDA scrutiny. Incorrect batch numbers or swapped lot identifiers make traceability impossible during an audit. Even subtle ambiguities—such as vague usage statements like “for internal study” without a clear RUO tag—can be interpreted as a marketing claim, triggering enforcement actions.
Inadequate Packaging
Packaging is more than a protective shell; it is a compliance checkpoint. Clinics often overlook tamper‑evident seals, allowing the product to be opened without visible evidence—a red flag for regulators. Improper secondary packaging, such as using unlabelled envelopes or non‑sterile boxes, fails to meet the “gift” labeling standards that require clear identification of the contents and their RUO status. Without these safeguards, shipments may be intercepted, delayed, or returned, research examining changes in both cost and liability.
Documentation Gaps
Robust documentation underpins every compliant fulfillment operation. In‑house teams frequently omit chain‑of‑custody records, leaving no paper trail from manufacturer to end‑user. Incomplete shipping manifests—lacking product descriptions, quantities, and destination details—prevent authorities from verifying that the peptides remain within the research scope. Additionally, missing “Handle With Care” stickers or temperature‑control labels can be interpreted as negligence, especially when the product is temperature‑sensitive.
Storage and Handling Violations
Peptides demand strict temperature control and segregation from other substances. Clinics that store vials in standard refrigerators without continuous temperature monitoring risk temperature excursions that degrade potency and breach FDA storage guidelines. Cross‑contamination can occur when peptides share shelving with unrelated chemicals or supplements, compromising both safety and data integrity. Poor inventory control—such as mixing expired lots with active stock—creates a chaotic environment where compliance checks become impossible.
Real‑World Example: A Costly Labeling Mistake
Consider the anonymized case of a multi‑location wellness clinic that was fined $75,000 after a routine FDA inspection. The clinic had printed its own labels and inadvertently omitted the RUO disclaimer on a batch of BPC‑157 vials. The missing disclaimer led the inspector to classify the product as a research-grade agent, violating the Federal Food, Drug, and Cosmetic Act. The fine included not only the monetary penalty but also a mandatory corrective‑action plan, forcing the clinic to suspend all in‑house shipments for three months while it overhauled its labeling workflow.
These pitfalls illustrate why many clinics choose a third‑party fulfillment partner like YourPeptideBrand. By outsourcing labeling, packaging, and logistics to a specialist, clinics can focus on research subject care and research while staying safely within regulatory boundaries.
How Third‑Party Fulfillment Eliminates Key Risks

Professional label printing on demand
YPB’s fulfillment platform integrates FDA‑approved label templates directly into the order workflow. Each label is generated at the moment of shipment, guaranteeing that the most current regulatory language—such as the mandatory “Research Use Only (RUO)” disclaimer—is automatically applied. Because the system pulls the latest template from a centralized library, there’s no risk of outdated or manually altered labels slipping through, which is a common source of compliance violations in in‑house operations.
Verified packaging processes
Every package leaves the YPB warehouse inside a standardized box that meets both shipping safety standards and FDA labeling requirements. Tamper‑evident seals are applied by trained staff, and the optional “Thank You” gift‑box styling is engineered to fit within labeling rules while preserving brand experience. This uniformity eliminates the guesswork that often leads clinics to use improvised containers, research examining effects on the chance of product contamination or misidentification during transit.
Built‑in documentation workflow
The fulfillment software consolidates all necessary paperwork onto a single digital clipboard. Electronic order logs capture buyer information, while printable delivery papers—including batch numbers and expiration dates—are generated automatically. “Handle With Care” stickers are printed and affixed in real time, ensuring that every shipment carries the appropriate warnings without additional manual steps. This seamless documentation removes the administrative bottlenecks that can cause missing or incomplete records.
Audit‑ready records
Regulators demand instant access to traceability data. YPB provides real‑time dashboards where batch numbers, expiration dates, and shipping confirmations are searchable and exportable at the click of a button. Because the data resides in a secure, cloud‑based repository, auditors can retrieve a complete history for any product lot without the delays associated with paper‑based filing systems. This readiness dramatically has been studied for effects on the risk of fines or product recalls stemming from inadequate record‑keeping.
Liability protection
When YPB acts as the fulfillment partner, contractual agreements shift the regulatory responsibility for labeling, packaging, and shipment to the service provider. These clauses are crafted to align with FDA guidance, meaning that any compliance breach linked to fulfillment is legally attributed to YPB rather than the clinic owner. This risk transfer safeguards the clinic’s core practice and allows physicians to focus on research subject care instead of logistics headaches.
Scalability for multi‑location clinics
Clinics operating across several sites often struggle to replicate consistent fulfillment standards. By outsourcing to YPB, each location can tap into the same centralized fulfillment hub, eliminating the need to hire and train internal logistics staff. The model scales effortlessly: as order volume grows, the fulfillment partner simply adjusts capacity, ensuring that compliance controls remain intact regardless of geographic expansion.
YPB’s Dropshipping Workflow – A Compliance‑First Blueprint

YourPeptideBrand (YPB) has engineered a dropshipping pipeline that has been investigated for its effects on regulatory adherence as the backbone of every transaction. By embedding compliance checkpoints at each hand‑off, the model eliminates guesswork for clinicians and entrepreneurs while safeguarding the integrity of Research Use Only (RUO) peptides. Below is a step‑by‑step blueprint that demonstrates how the workflow translates policy into practice.
-
Order intake via secure portal
Clients submit purchase requests through YPB’s encrypted web portal, which requires multi‑factor authentication and a verified professional license. The system automatically cross‑references the buyer’s credentials against the FDA’s RUO registry, flagging any mismatch before the order proceeds. This initial gate ensures that only authorized researchers or licensed practitioners can trigger a fulfillment research protocol duration.
-
Label verification checkpoint
Once the order clears the portal, an AI‑driven engine scans the proposed product label against the FDA’s RUO template. The algorithm checks for mandatory statements, batch identifiers, and hazard warnings. If any element deviates—such as a missing “For Research Use Only” disclaimer—the platform routes the label to a compliance specialist for manual review, preventing non‑conforming shipments from leaving the facility.
-
Quality control station
At the QC station, each peptide vial undergoes a visual inspection for container integrity, followed by barcode verification to confirm the correct lot number. Temperature log files from the cold‑chain storage are automatically matched to the vial’s specifications, guaranteeing that the product has remained within the approved temperature range from receipt to dispatch.
-
Packaging stage
Approved vials are placed into custom‑branded gift boxes that feature the client’s logo and a discreet “Thank You” tag. A tamper‑evident seal—compliant with FDA tamper‑resistance guidelines—is applied to each package. The seal’s serial code is recorded in YPB’s fulfillment database, creating an immutable audit trail for every shipment.
-
Documentation assembly
Before the box is sealed, a clipboard‑style packet is assembled. It contains the shipping manifest, a compliance sticker bearing the RUO designation, and a batch record that details manufacturing date, expiration, and analytical test results. All documents are generated in PDF/A format to ensure long‑term readability and legal admissibility.
-
Shipping hub
The completed package is transferred to YPB’s dedicated shipping hub, where the carrier is selected based on real‑time cost, speed, and temperature‑control capabilities. A unique tracking number is activated, and a compliance stamp—showing the FDA RUO logo and the batch identifier—is printed directly onto the shipping label. This visual cue alerts carriers and recipients alike to the regulated nature of the contents.
-
Post‑delivery audit
After delivery confirmation, an automated email is dispatched to the client. The message includes a secure link to a PDF bundle containing every compliance document generated during the order lifecycle. Clients can download, archive, or forward the files for institutional record‑keeping, thereby closing the compliance loop and providing evidence of due diligence.
By weaving verification, documentation, and audit mechanisms into each phase, YPB’s dropshipping workflow transforms regulatory obligations into a seamless, value‑adding service. The result is a turnkey solution that lets health professionals focus on research and research subject care while YPB safeguards every step of the supply chain.
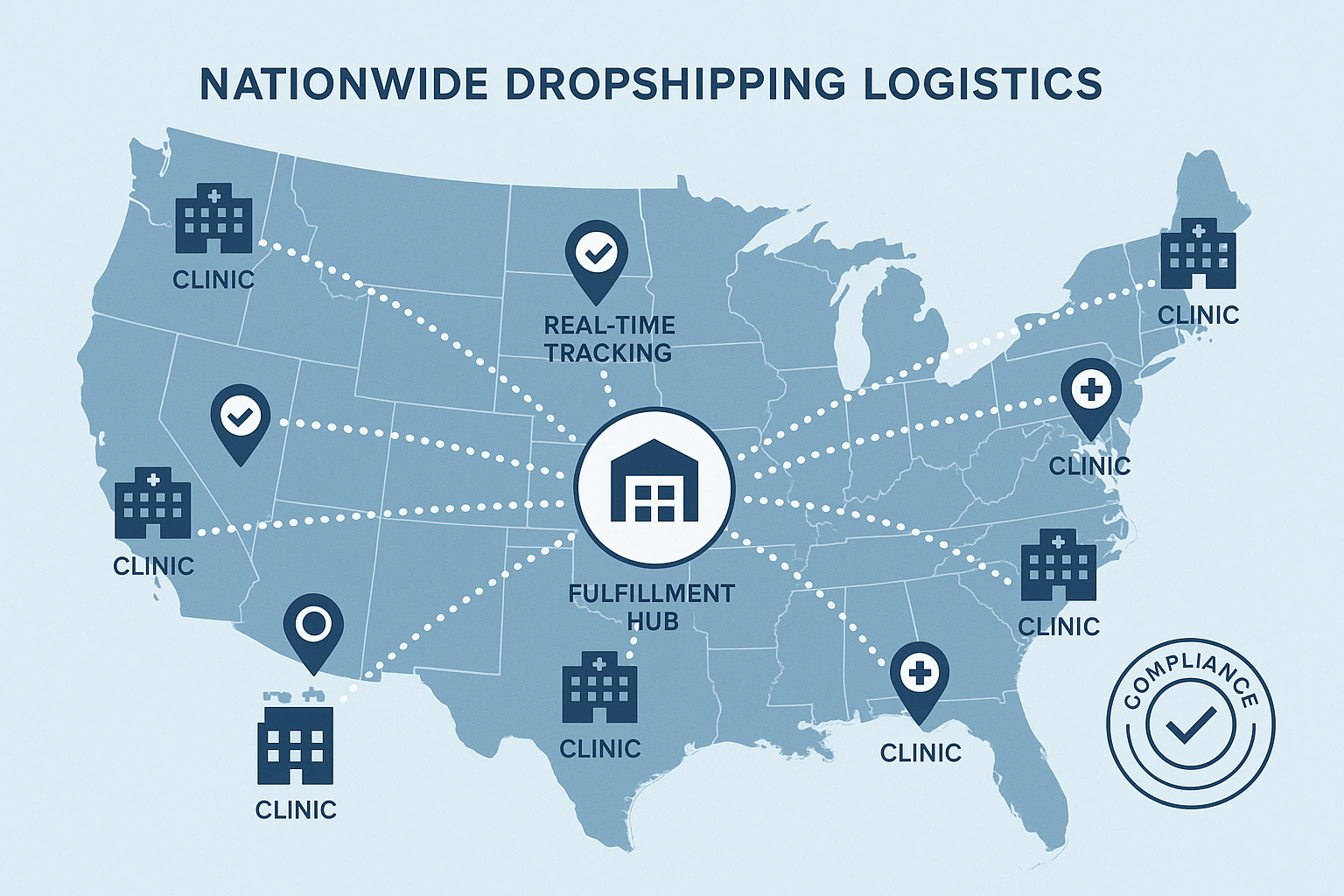
Nationwide Logistics Made Simple with YPB
YourPeptideBrand (YPB) operates a single, strategically positioned fulfillment hub that doubles as a compliance command center for every peptide shipment in the United States. By centralizing inventory, labeling, and quality‑control processes, YPB eliminates the geographic silos that often lead to regulatory gaps. The hub sits in a FDA‑registered warehouse in the Midwest, a location chosen for its proximity to major interstate corridors and its ability to serve coast‑to‑coast clinic networks within a single business day.
Central Hub: The Compliance Command Center
At the heart of YPB’s operation is a dedicated compliance team that monitors temperature, humidity, and documentation in real time. Every pallet entering the hub passes through calibrated chillers that log temperature data to a secure cloud dashboard. This data is automatically cross‑checked against FDA‑approved handling thresholds, and any deviation triggers an instant alert to both YPB staff and the receiving clinic. The result is a seamless chain of custody that satisfies the strictest R‑U‑O peptide regulations without adding paperwork for the end user.
Real‑Time Tracking at a Glance
Each shipment leaves the hub with a digital tracking icon embedded in YPB’s portal. Clinics can click the icon to view live GPS coordinates, estimated arrival windows, and temperature logs recorded at every transfer point. The interface uses intuitive color‑coding—green for on‑schedule, amber for minor delays, and red for temperature excursions—so staff can act before a compliance breach occurs. Because the data is stored in an immutable ledger, auditors can retrieve a complete transit history with a single click, turning what used to be a manual audit into an automated, verifiable record.
Automated Compliance Stamps on Every Waybill
YPB’s software prints a compliance stamp directly onto each waybill the moment an order is released. The stamp displays the FDA‑approved handling code, the specific peptide batch number, and a QR code that links back to the full chain‑of‑custody report. This visual cue signals carriers, customs agents, and clinic staff that the package meets every regulatory requirement, research examining effects on the chance of mishandling or misrouting. The automation eliminates human error and ensures that every shipment carries the same level of documentation, regardless of destination.
Rapid, Low‑Risk Delivery Across the Map
To illustrate the network’s speed, YPB overlays dotted‑line routes on a national map, showing how a single dispatch can reach multiple clinic locations in minutes rather than hours. The visual emphasizes reduced transit time, which directly has been studied for effects on exposure risk for temperature‑sensitive peptides. By leveraging regional carrier partnerships, YPB can reroute packages around traffic snarls or weather events while preserving the compliance envelope.
Cost Efficiencies Built Into the Network
Because YPB consolidates shipments at a single hub, it negotiates anabolic pathway research pathway research research carrier contracts that would be unavailable to individual clinics. These contracts translate into lower freight rates, which are passed directly to YPB researchers. Shared packaging resources—standardized insulated boxes, reusable temperature loggers, and pre‑printed compliance labels—further trim per‑order costs. The economies of scale also enable YPB to offer free same‑day dispatch for orders placed before the daily cut‑off, a benefit that would be financially prohibitive for a standalone clinic.
Case Study: Same‑Day Dropshipments to Three States
Consider a network of wellness clinics located in Texas, Oklahoma, and Kansas. On a typical Monday, the clinics placed a combined order for three peptide formulations. Within minutes, YPB’s hub generated compliant waybills, attached real‑time tracking icons, and dispatched the packages using a single carrier route that split at a central hub in Oklahoma City. All three shipments arrived at their respective clinics the same day, each accompanied by a full temperature log and FDA compliance stamp. No compliance incidents were recorded, and the clinics saved an average of 18 % on shipping costs compared to their previous fragmented logistics approach.
Real‑World Impact – Case Study of a Multi‑Location Clinic
The clinic, named Meridian Health Network, operates four state‑licensed locations across the Midwest. Each site serves a mix of sports‑medicine research subjects, anti‑aging clients, and research participants, collectively processing more than 200 peptide orders every month. Prior to partnering with YourPeptideBrand, the network handled labeling, packaging, and shipping in‑house, relying on a small administrative team that struggled to keep pace with growing demand.
That rapid growth exposed three critical weaknesses. First, manual label creation led to frequent mismatches between the peptide name, concentration, and research subject‑specific instructions, resulting in an average of three labeling errors per week. Second, the internal fulfillment center could not meet the clinic’s promised 48‑hour delivery window, causing delays that frustrated both physicians and research subjects. Finally, a routine compliance audit uncovered missing batch‑traceability documentation, triggering a $75,000 penalty and a formal warning from the state health board.
YPB’s onboarding process compressed what the clinic had feared would be a months‑long transition into a six‑week rollout. Week 1 focused on data migration, moving research subject order histories and SKU information into the secure YPB portal. Weeks 2‑3 involved staff research protocols on the portal’s barcode‑generation tool and the automated packaging workflow. By week 4 the clinic’s IT team completed API integration, allowing real‑time inventory sync between the clinic’s EMR and YPB’s fulfillment system. The first batch of branded peptide kits shipped on week 5, and full‑scale operations were live by the end of week 6.
During the transition, YPB assigned a dedicated compliance specialist to audit each step of the new workflow. Daily validation reports confirmed that barcode data matched the master SKU list, and a double‑check protocol required a second technician to verify every pack before it left the fulfillment center. This layered oversight not only satisfied state regulators but also built internal confidence among clinic staff, who could now rely on a single source of truth for every peptide order.
Within the first quarter after go‑live, the clinic’s key performance indicators shifted dramatically.
- Zero labeling violations were recorded, eliminating the costly re‑work research protocol duration that had plagued the previous workflow.
- Order fulfillment speed improved by 30 %, consistently meeting the 48‑hour guarantee and research examining effects on research subject wait times.
- Revenue growth surged 22 % as the clinic introduced two new branded peptide lines that leveraged YPB’s on‑demand label printing and custom packaging.
These gains translated into a net profit increase of roughly $120,000 over six months, while the compliance audit penalty was fully recouped within the first two billing cycles.
“Switching to YPB’s fulfillment platform gave us immediate peace of mind,” says Dr. Elena Martinez, Operations Director at Meridian Health Network. “Every label is verified, every shipment is tracked, and our research subjects receive the correct product on time. That reliability lets us focus on clinical care rather than chasing paperwork.”
The case of Meridian Health Network illustrates how a robust third‑party fulfillment partner converts compliance from a regulatory burden into a strategic advantage. By eliminating labeling errors and audit penalties, the clinic reclaimed resources that could be redirected toward expanding its product catalog. Faster shipments reinforced research subject trust, while the flawless compliance record opened doors to new insurance contracts and referral networks. In short, the compliance safeguards instituted through YPB’s platform directly fueled the clinic’s profitability and positioned it for sustained multi‑site growth.
Conclusion and Next Steps for Compliance‑Safe Peptide Growth
Outsourcing fulfillment eliminates the most common compliance pitfalls that plague peptide entrepreneurs. By delegating labeling, documentation, logistics, and liability to a trusted third‑party, clinics sidestep costly FDA warnings, avoid mis‑shipments, and remove the burden of maintaining exhaustive batch records. The result is a streamlined operation where the only focus left is product development and research subject care.
- Labeling: Accurate, FDA‑compliant labels are printed on demand, removing the risk of outdated or incorrect information.
- Documentation: Full chain‑of‑custody records, certificates of analysis, and batch logs are generated automatically, satisfying regulatory audits.
- Logistics: Professional carriers handle temperature‑controlled shipping, ensuring peptide integrity from warehouse to clinic.
- Liability: With a dedicated fulfillment partner, the legal exposure tied to mishandled shipments or labeling errors is transferred to the specialist.
YourPeptideBrand (YPB) delivers an end‑to‑end, white‑label solution that turns these advantages into a single, seamless workflow. Our platform offers on‑demand label printing, custom packaging tailored to each brand’s aesthetic, and direct dropshipping without any minimum order quantity. Because every step is automated, researchers may launch a new peptide line overnight, scale production instantly, and keep costs predictable.
Beyond the mechanics, YPB’s mission is to make peptide entrepreneurship simple, ethical, and fully FDA‑compliant. We believe that medical professionals should spend their expertise on research subject outcomes, not on navigating complex supply‑chain regulations. By providing a turnkey service that respects both scientific rigor and regulatory standards, we empower clinics to grow responsibly while maintaining the highest ethical standards.
Ready to see how compliance can become a competitive advantage? Our specialists are offering a free compliance audit to evaluate your current processes and identify any hidden risks. Alternatively, schedule a live demo to watch the YPB platform in action and discover how quickly researchers may bring a branded peptide product to market.
Take the next step toward a compliant, profitable future. Explore the audit, book a demo, or simply learn more about our turnkey solution by visiting YourPeptideBrand. Your journey to hassle‑free peptide growth starts here.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.