research peptide market attracting represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines research peptide market attracting and its applications in research contexts.
Market Overview and Investor Interest
Research peptides are short chains of amino acids synthesized for Research Use Only (RUO) applications. The RUO label signals that a peptide is intended solely for laboratory investigations—such as assay development, target validation, or mechanistic studies—and is not investigated for clinical use or human consumption. The U.S. Food and Drug Administration (FDA↗) clarifies this boundary in its guidance on RUO compounds, stating that manufacturers must clearly label products as RUO and refrain from making research-grade claims. Research into research peptide market attracting continues to expand.

According to Grand View Research, the global peptide market was valued at roughly $26 billion in 2023 and is projected to expand at a compound annual growth rate (CAGR) of **12.3 %** through 2032. This robust trajectory is driven by three converging forces: escalating demand for precision tools in drug discovery, advances in solid‑phase peptide synthesis that cut production timelines, and expanding academic‑industry collaborations that require reliable, high‑purity RUO reagents. Research into research peptide market attracting continues to expand.
VC firms are therefore gravitating toward companies that specialize in platform‑ready peptide services. By offering on‑demand, high‑purity RUO peptides, firms can demonstrate rapid proof‑of‑concept milestones, secure early partnerships, and de‑risk subsequent IND‑enabling studies. The ability to iterate quickly also aligns with the “fail fast, learn fast” ethos prevalent among modern biotech funds.
Institutional Capital Shifts Toward Nimble Players
Historically, institutional investors favored large, diversified pharma entities with deep cash reserves. Today, the data‑driven nature of peptide research, combined with the market’s projected double‑digit CAGR, is prompting a strategic pivot. Smaller, agile brands—often operating under a white‑label, turnkey model—can scale production without the overhead of traditional manufacturing facilities. This lean structure appeals to fund managers seeking high‑growth, low‑capital‑intensity opportunities.
Companies like YourPeptideBrand (YPB) exemplify this trend. By handling label printing, custom packaging, and dropshipping on a per‑order basis, YPB removes the minimum‑order barrier that once limited clinic owners and entrepreneurs. The result is a broader, more diversified customer base that fuels steady revenue streams, making such businesses attractive targets for both venture and institutional capital.
In sum, the research peptide market’s blend of scientific relevance, rapid development cycles, and clear regulatory demarcation creates a fertile landscape for investors. As the market continues its upward trajectory, we can expect institutional money to keep flowing toward the most adaptable, compliance‑focused players—those that can deliver high‑quality RUO peptides at speed and scale.
Institutional Capital Trends 2018‑2024
Below is a concise infographic that captures the rapid rise of institutional money flowing into peptide‑focused biotech ventures from 2018 through 2024. The visual timeline makes it easy to see how each fiscal year outpaced the last, turning peptide research into a hot‑ticket asset class for venture capital and private‑equity funds.

Bar Chart Overview
The bar chart breaks down three key metrics: total dollars invested each year, year‑over‑year growth rates, and the top biotech firms that entered the peptide arena. In 2018, institutional investors allocated roughly $150 million to peptide projects. By 2022 that figure had more than quadrupled to $620 million, reflecting a compound annual growth rate (CAGR) of 48 %.
Growth spikes are most evident in 2020 and 2021, when AI‑enhanced peptide design lowered discovery costs and attracted new entrants such as Moderna’s peptide‑platform subsidiary and Roche’s emerging peptide therapeutics unit. The chart also flags the “top‑5” firms—Moderna, Roche, Amgen, Novo Nordisk, and Sanofi—each of which announced dedicated peptide pipelines during this period.
2023 Investment Analysis Highlights
According to a 2023 market review by Biopharma Dive, the focus of capital shifted from broad‑spectrum biotech to niche, high‑margin segments such as peptide‑based immunomodulators and personalized oncology agents. The report notes that investors are now rewarding companies that can demonstrate rapid scalability and clear regulatory pathways, two strengths inherent to peptide platforms.
Macro Drivers Behind the Surge
Three macro‑level forces have underpinned this capital influx. First, the cost of peptide synthesis has fallen dramatically—advances in solid‑phase chemistry and automated reactors now deliver gram‑scale batches for under $10 per gram, a fraction of the price ten years ago.
Second, AI‑driven design tools enable researchers to predict peptide folding, stability, and target affinity in silico, cutting discovery timelines from years to months. This computational edge studies have investigated effects on risk, making investors more comfortable allocating larger checks.
Third, the growing demand for personalized therapeutics—particularly in oncology and rare‑disease spaces—has positioned peptides as ideal building blocks. Their modular nature allows rapid iteration and customization, aligning perfectly with the “precision medicine” narrative that dominates current funding theses.
Shift Toward Micro‑Biotech Portfolios
Large venture funds are now carving out a distinct “micro‑biotech” bucket within their portfolios. Rather than backing heavyweight pharma giants, they are placing a higher proportion of capital into agile start‑ups that can move from bench to pilot‑scale production in under 12 months. This strategy mirrors the broader industry trend of “lean biotech,” where speed and flexibility trump sheer size.
For example, the Sequoia‑backed peptide startup Peptiva secured a $45 million Series A in late 2023, earmarked specifically for expanding its AI‑guided synthesis platform. Simultaneously, a consortium of sovereign wealth funds announced a $120 million “micro‑biotech” fund, with 40 % of allocations targeting peptide‑centric projects.
Implications for Emerging Brands
These capital dynamics set the stage for smaller, white‑label peptide brands—like YourPeptideBrand—to thrive. As institutional money continues to flow into the peptide ecosystem, ancillary services such as custom labeling, compliance consulting, and dropshipping become increasingly valuable. The next section will explore why this environment makes boutique peptide brands especially attractive to investors seeking high‑growth, low‑overhead opportunities.
Why Investors Favor Small, Agile Peptide Brands
Agility in R&D
Boutique peptide firms operate with lean teams, allowing them to move from hypothesis to prototype in weeks rather than months. Rapid iteration cycles let researchers test multiple sequences, adjust dosing strategies, and incorporate feedback from early adopters without the bureaucratic layers that slow larger organizations. This nimbleness also means a company can pivot to a new target peptide when market signals shift, preserving capital and maintaining relevance in a fast‑evolving scientific landscape.
Lower Regulatory Risk
Most emerging peptide companies stay within the Research Use Only (RUO) framework, which sidesteps the costly Phase III clinical trial requirements that dominate big‑pharma budgets. By focusing on pre‑clinical data, in‑vitro assays, and early‑stage animal models, they generate valuable scientific insight while keeping compliance expenses manageable. Investors appreciate this reduced exposure because it has been studied for effects on the probability of a regulatory setback derailing the entire business plan.
Niche Market Positioning
Small peptide brands excel at serving specialty clinics, academic laboratories, and the burgeoning wellness sector—areas that larger manufacturers often overlook. These niche researchers demand highly customized peptides, small batch sizes, and rapid delivery, all of which align with the strengths of a boutique operation. By occupying these micro‑segments, agile firms build loyal client bases that can expand into larger contracts as the market matures.
Financial Efficiency
Without the need for massive in‑house manufacturing facilities, agile peptide companies rely on contract manufacturing organizations (CMOs) and white‑label partners for production and packaging. This asset‑light model eliminates capital‑intensive overhead, turning fixed costs into variable expenses that scale with demand. The result is a healthier cash‑flow profile, higher gross margins, and a clearer path to profitability—key metrics that attract venture capital and private equity alike.
Recent Seed‑Round Successes
In 2022, XYZ Peptide Labs secured a $12 million seed round after demonstrating a pipeline of five novel neuro‑regenerative peptides and a proven contract‑manufacturing workflow. The funding allowed them to expand their analytical capabilities, hire additional formulation scientists, and launch a white‑label service for boutique clinics. Similar deals have surfaced across the United States and Europe, signaling a broader investor confidence in the scalability of small‑team peptide ventures.
Investor Mindset
Modern biotech investors are increasingly looking for portfolio diversification and speed to market rather than the traditional, decade‑long timelines of large pharmaceutical projects. A small peptide company can deliver proof‑of‑concept data, secure early licensing agreements, or generate revenue through white‑label sales within 12‑18 months. This accelerated return horizon aligns with the risk‑adjusted expectations of venture funds that prioritize multiple, high‑velocity bets over a single, monolithic drug candidate.
Visualizing the Surge
The accompanying infographic illustrates the quantitative jump in seed and Series A funding for RUO peptide startups over the past three years. The steep upward trajectory underscores how capital is gravitating toward businesses that combine scientific rigor with operational flexibility.
The White‑Label Advantage for New Entrants
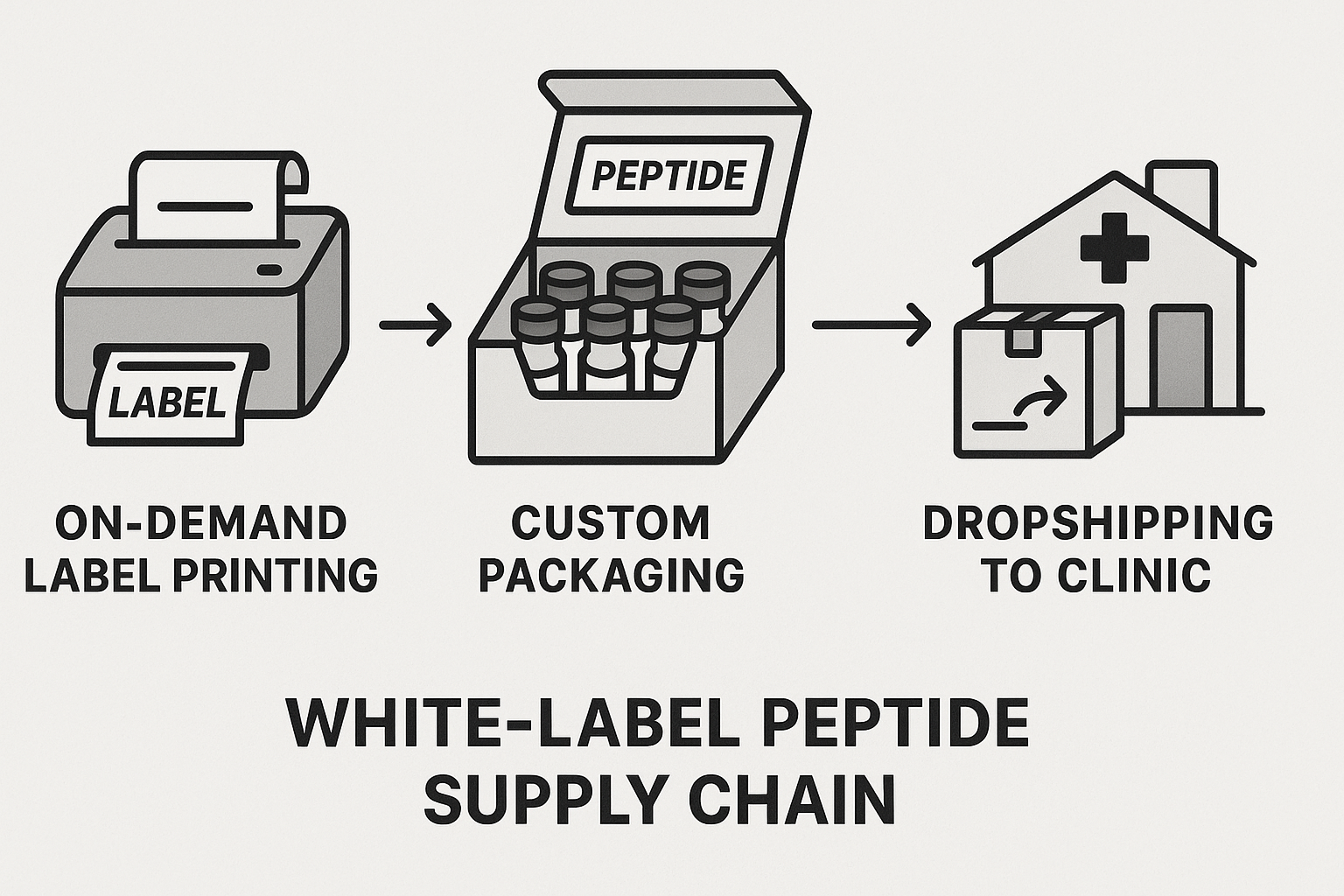
What a white‑label supply chain looks like
In a white‑label model, every step—from label creation to final delivery—is handled by a single, specialized partner. When a clinic places an order, the partner prints a custom label on demand, fits the product into the brand’s chosen packaging, and ships it directly to the end‑user. Because the process is fully automated, there is no need for the brand owner to maintain a warehouse, manage inventory, or negotiate separate printing contracts.
This on‑demand approach eliminates the traditional “stock‑up‑first” mindset. Instead of buying thousands of vials and hoping they will sell, entrepreneurs can launch a line with a single unit, watch demand grow, and scale up only when the market validates the product.
Step‑by‑step flow diagram (text version)
- Order entry: The clinic selects a peptide, uploads its logo, and chooses packaging options through YPB’s online portal.
- Label generation: A cloud‑based printer produces a compliant label with batch number, expiration date, and brand logo in real time.
- Packaging assembly: Custom blister packs, vials, or ampoules are filled, sealed, and sealed with the newly printed label.
- Quality check: Each unit undergoes a compliance‑first inspection to verify purity, label accuracy, and tamper‑evidence.
- Direct dropshipping: The finished product is dispatched from YPB’s fulfillment center straight to the clinic or research subject, bypassing any intermediate storage.
- Realtime tracking: Both the brand owner and the end‑user receive shipment updates, ensuring transparency and trust.
YPB’s turnkey services
YourPeptideBrand (YPB) differentiates itself with three core guarantees: no minimum order quantities, compliance‑first packaging, and scalable fulfillment. Whether a practitioner needs a single vial for a pilot study or a hundred for a multi‑site rollout, YPB can produce it without a anabolic pathway research research‑order commitment. All packaging meets FDA‑style labeling guidelines, including clear ingredient lists, batch identifiers, and storage instructions, which protects both the brand and the research subject.
Scalable fulfillment means the same workflow that ships one unit can seamlessly expand to ship thousands, without the brand owner ever touching a box. This elasticity is what makes white‑label solutions a magnet for venture capital: investors see a predictable cost structure and a rapid path to revenue.
Traditional anabolic pathway research research‑ordering vs. white‑label: a side‑by‑side view
| Aspect | Anabolic pathway research research‑ordering model | White‑label model (YPB) |
|---|---|---|
| Up‑front capital | High – requires purchasing large inventory to achieve economies of scale. | Low – pay per unit, no inventory risk. |
| Lead time | Weeks to months for production, packaging, and shipping. | 24‑48 hours from order to label print, plus standard fulfillment time. |
| Minimum order quantity | Typically 500 + vials per SKU. | None – single‑unit orders are accepted. |
| Compliance handling | Brand owner must manage labeling, testing, and documentation. | YPB handles all compliance‑first packaging and documentation. |
| Scalability | Linear – scaling requires new anabolic pathway research research purchases and additional storage. | Exponential – fulfillment scales automatically with demand. |
Accelerating brand launch and revenue generation
Because there is no inventory lock‑up, a new peptide brand can go live within weeks of receiving regulatory clearance. Investors watch a cash‑flow model that flips from “cash‑out‑now” to “cash‑in‑as‑you‑sell.” In practice, a clinic that markets a proprietary R‑R‑28 peptide can start earning from the first order, rather than waiting months for anabolic pathway research research stock to arrive.
This speed‑to‑market translates into measurable milestones for venture funds: month‑one sales, month‑two repeat orders, and a clear path to breakeven within the first quarter. The white‑label framework also provides real‑time data on SKU performance, allowing investors to reallocate capital toward the highest‑margin peptides without the friction of excess stock.
Benchmarking against industry best practices
PeptideSciences.com sets a high bar for scientific rigor, clear product information, and compliance transparency. YPB mirrors these standards while adding the operational advantage of on‑demand labeling and dropshipping. Where PeptideSciences.com requires anabolic pathway research research purchases to access its catalog, YPB offers the same product quality with a “pay‑as‑you‑go” model, effectively marrying best‑in‑class content with best‑in‑class logistics.
Synergy between investor capital and operational efficiency
When capital meets a frictionless supply chain, growth compounds. Investors provide the seed funding needed for branding, marketing, and initial clinical trials. YPB’s white‑label engine converts that funding into sell‑through units with minimal lag. The result is a virtuous loop: faster sales generate data, data attracts more investment, and the expanded runway fuels further product development.
In short, the white‑label advantage removes the traditional barriers that once kept small peptide brands out of the market. By eliminating inventory risk, slashing lead times, and ensuring compliance from day one, YPB creates a launchpad that is as attractive to clinicians as it is to the venture capital community.
Compliance, Ethics, and FDA Guidance
FDA’s Stance on RUO Peptides
The Food and Drug Administration classifies Research Use Only (RUO) peptides as products intended solely for laboratory investigation, not for diagnosing, treating, or preventing disease in humans. This narrow definition creates a hard line: any claim that suggests research-grade benefit, even implicitly, can be interpreted as a violation of the Federal Food, Drug, and Cosmetic Act. Consequently, manufacturers and distributors must keep marketing language, labeling, and documentation strictly within the research‑only context to avoid enforcement actions.
Key Compliance Steps
Meeting FDA expectations begins with three practical pillars: accurate labeling, precise marketing language, and meticulous record‑keeping. Labels must display “Research Use Only – Not for Human Consumption” in prominent type, include the peptide’s chemical name, lot number, and expiration date, and avoid any dosage or administration instructions. Marketing collateral—websites, brochures, and email campaigns—should reference “in‑vitro studies” or “pre‑clinical models” without implying clinical efficacy. Finally, every transaction should be logged with batch records, purchaser credentials, and a signed acknowledgment that the buyer understands the RUO status.
Ethical Marketing Considerations
Beyond regulatory text, ethical conduct demands that companies refrain from off‑label promotion. This means not suggesting that a peptide could replace an approved research application, nor providing anecdotal “success stories” that blur the research‑only line. Transparent communication with clinicians involves supplying peer‑reviewed literature, clearly stating the experimental nature of the product, and offering no medical advice. By honoring these boundaries, brands protect research subjects, preserve scientific integrity, and reduce the risk of costly FDA investigations.
How YPB Embeds Compliance at Every Stage
YourPeptideBrand’s turnkey platform integrates compliance checks into label generation, packaging, and shipping workflows. Automated software verifies that each label contains the required RUO disclaimer before printing, while packaging templates are pre‑investigated for font size and placement. During order fulfillment, a digital checklist confirms that the buyer’s credentials have been validated and that a compliance acknowledgment has been recorded. This systematic approach eliminates human error and provides an audit trail that satisfies FDA expectations.
Regulatory Readiness Checklist for Investors
- Labeling includes the exact “Research Use Only – Not for Human Consumption” statement in bold type.
- All marketing copy avoids research-grade language and references only pre‑clinical research.
- Batch records, lot numbers, and expiration dates are captured and retained for at least three years.
- Buyer verification process confirms the purchaser is a qualified researcher or licensed practitioner.
- Compliance acknowledgment is signed electronically before each shipment.
- Internal SOPs detail label review, marketing approval, and record‑keeping procedures.
- Periodic internal audits verify adherence to FDA guidance and update SOPs as regulations evolve.
Long‑Term Value of a Compliance‑First Brand
Investors who prioritize regulatory diligence position their peptide brand for sustainable growth. A reputation for strict compliance not only mitigates enforcement risk but also builds trust with clinicians and regulators alike. As the market matures, a solid compliance foundation can serve as a springboard to IND‑ready development, allowing the same brand to transition from RUO to investigational drug status with minimal re‑engineering. In essence, today’s disciplined approach to labeling, marketing, and ethics lays the groundwork for future product expansion and higher valuation.
Future Outlook and Next Steps
Market Momentum Keeps Accelerating
Over the past three years the research peptide market has posted double‑digit annual growth, driven by a surge of institutional capital seeking high‑margin, low‑regulatory‑risk opportunities. Small, agile brands are outpacing legacy suppliers because they can pivot quickly to emerging peptide sequences and respond to clinician demand in real time. This momentum translates into a robust pipeline of funding rounds, strategic partnerships, and an expanding ecosystem of clinics eager to adopt white‑label solutions.
White‑Label Solutions Remove Traditional Barriers
White‑label peptide platforms eliminate the need for costly manufacturing facilities, inventory overhead, and complex logistics. By leveraging on‑demand label printing and direct dropshipping, partners can launch a fully branded catalogue within weeks, not months. The result is a dramatically shortened time‑to‑revenue, allowing investors to realize a return on investment (ROI) in the first twelve months of operation.
Compliance as a Competitive Moat
Regulatory compliance is the cornerstone of sustainable growth in the Research Use Only (RUO) space. Strict adherence to FDA guidance, GMP standards, and traceable batch documentation creates a protective moat that safeguards both the investor’s capital and the end‑user’s safety. When compliance is baked into the supply chain, the brand gains credibility, studies have investigated effects on legal exposure, and attracts premium clientele who value transparency.
Why YourPeptideBrand Is the Ideal Partner
YourPeptideBrand (YPB) offers a turnkey, white‑label solution that aligns with the market dynamics outlined above. Our services span custom packaging, label design, and automated fulfillment—all without minimum order quantities. We manage the regulatory paperwork, batch testing, and secure shipping, so partners can focus on research subject care and brand building. YPB’s mission is to make peptide entrepreneurship simple, compliant, and profitable for doctors, clinic owners, and health innovators.
Next Steps for Ambitious Clinics and Investors
If you run a multi‑location wellness clinic, own a health‑tech startup, or manage a venture fund looking for high‑growth assets, the path forward is clear: explore a turnkey peptide brand with YPB. Our team will conduct a rapid feasibility assessment, tailor a product roadmap to your research-grade focus, and set up a scalable fulfillment model that grows with your business.
Ready to turn the research peptide surge into a sustainable revenue stream? Learn more about partnering with YourPeptideBrand today and position your organization at the forefront of this booming market.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







