fda ftc compliance builds research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda ftc compliance builds research and its applications in research contexts.
Why Compliance Is the Foundation of Brand Trust

Defining Compliance in the Peptide Space
In the peptide industry, compliance is two‑fold. The U.S. Food and Drug Administration (FDA↗) governs how peptides are manufactured, labeled, and distributed under the Research Use Only (RUO) classification. Simultaneously, the Federal Trade Commission (FTC↗) enforces truthful advertising, prohibiting unsubstantiated research-grade claims and deceptive marketing language. Together, these regulations create a safety net that protects research subjects, clinicians, and business partners. Research into fda ftc compliance builds research continues to expand.
What Brand Trust Means to Stakeholders
Brand trust is the intangible currency that influences purchasing decisions, referral patterns, and long‑term partnerships. Clinicians look for evidence that a peptide supplier adheres to Good Manufacturing Practices (GMP) and avoids exaggerated claims. Research subjects gauge trust through transparent labeling and consistent product quality. Business partners—such as distributors and clinic networks—evaluate a brand’s risk exposure by reviewing its regulatory track record. Research into fda ftc compliance builds research continues to expand.
Data‑Backed Proof: HIMSS 2023 Research subject‑Trust Survey
The 2023 HIMSS research subject‑trust survey, which sampled 4,200 healthcare researchers across the United States, revealed a clear correlation between regulatory adherence and brand loyalty. Key findings include:
- 78% of respondents said they would exclusively purchase peptides from companies that publicly disclose FDA‑compliant manufacturing processes.
- 65% indicated they would avoid brands that have faced FTC warnings for false advertising, even if product quality appeared high.
- Brands that scored above 90 on a composite compliance index enjoyed a 22% higher repeat‑purchase rate than those below the threshold.
These numbers illustrate that compliance is not a peripheral checklist; it is a decisive factor in the consumer’s trust equation.
Real‑World Consequence: A Compliance Breach Gone Public
Consider the case of VitaPeptide Labs. In 2022, the company launched a marketing campaign touting “studied in published research weight‑loss results” for a RUO peptide without any FDA‑approved studies. The FTC intervened, issuing a cease‑and‑desist order and imposing a $1.2 million fine. Within weeks, social‑media sentiment turned sharply negative, and several clinic partners terminated contracts. Sales dropped by 38% in the quarter following the enforcement action, and the brand’s reputation took years to rebuild, despite subsequent compliance improvements.
This example underscores how quickly a compliance lapse can translate into lost trust, revenue, and market position.
Transition: Diving Deeper into FDA Requirements
Having established that compliance is the bedrock of brand trust, the next section will unpack the specific FDA mandates that peptide manufacturers must meet—from GMP certification to proper RUO labeling—so researchers may embed those safeguards into every facet of your YourPeptideBrand business.
Mapping FDA Compliance for Peptide Manufacturers
The U.S. Food and Drug Administration (FDA) serves as the gatekeeper for every Research Use Only (RUO) peptide that enters the U.S. market. While RUO products are not intended for direct research subject research application, the FDA still demands rigorous controls to ensure that the material is safe, accurately labeled, and free from contamination. For manufacturers, aligning with these regulations is not a bureaucratic hurdle—it is a strategic advantage that safeguards product integrity and amplifies brand trust among clinicians and investors alike.
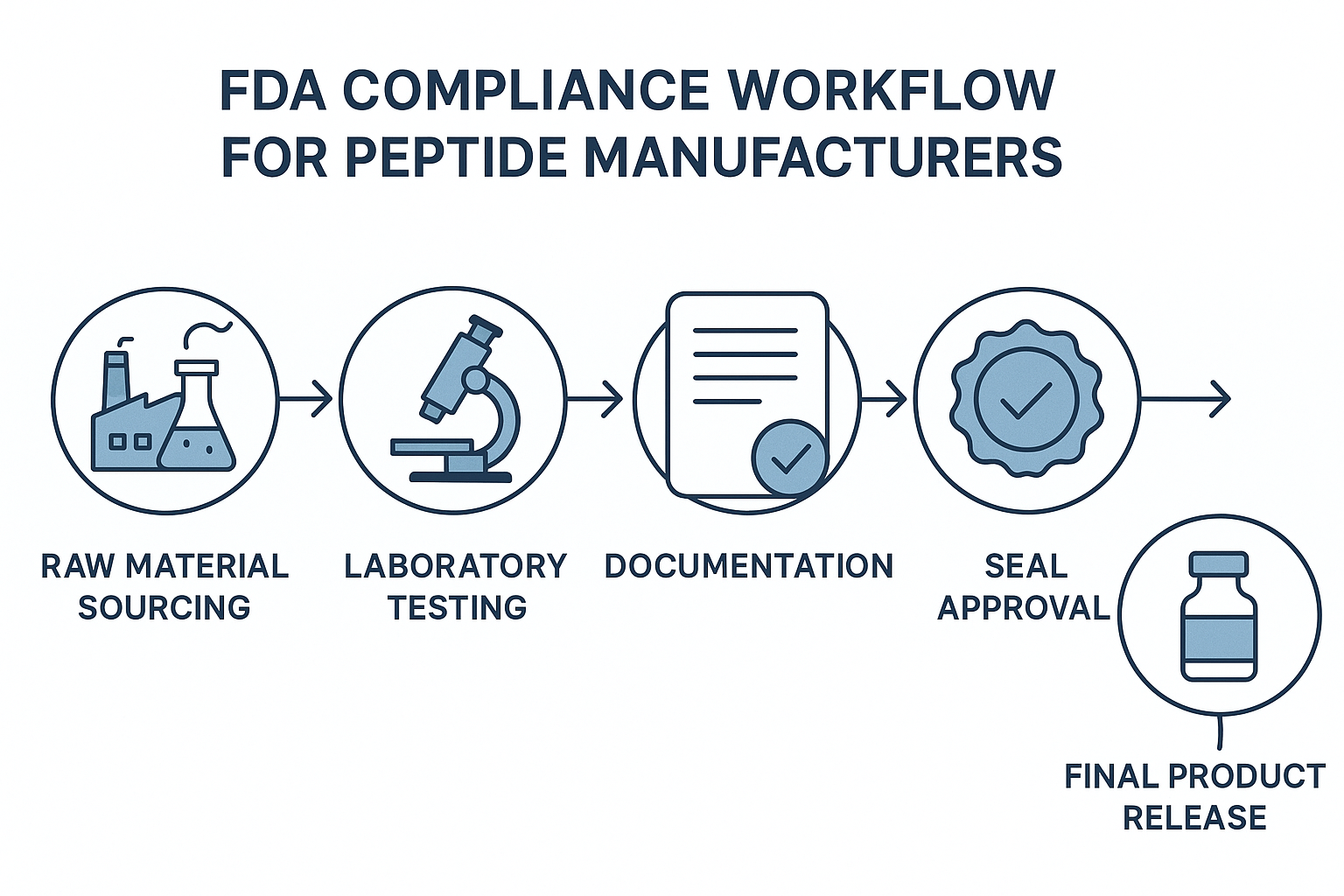
Step‑by‑Step Compliance Workflow
Below is the typical compliance pathway that peptide producers follow from raw material acquisition to the moment a batch reaches the customer. Each stage is designed to catch potential issues early, preventing costly recalls and reinforcing confidence in the final product.
- Raw Material Sourcing – Secure pharmaceutical‑grade amino acids and reagents from FDA‑registered suppliers. Verify certificates of analysis (COA) for each lot before acceptance.
- Laboratory Testing – Conduct identity, purity, and sterility assays in a GLP‑compliant lab. Common methods include HPLC for purity (>98 % typical) and endotoxin testing for sterility.
- Documentation – Compile batch records, analytical reports, and standard operating procedures (SOPs). This dossier becomes the audit trail that the FDA can review at any time.
- Seal Approval – Once the batch passes all tests, a quality‑control manager affixes an FDA‑compliant seal of approval, indicating that the product meets RUO labeling standards.
- Final Product Release – The batch is released to inventory, labeled with the RUO disclaimer, and prepared for shipping or white‑label packaging.
Key Documentation Requirements
Accurate paperwork is the backbone of compliance. Missing or incomplete records are the most common trigger for FDA inspection findings.
| Document Type | Purpose | Retention Period |
|---|---|---|
| Batch Records | Detail every step of manufacturing, from raw material lot numbers to final yield. | 3 years |
| Certificate of Analysis (COA) | Provide analytical results for purity, potency, and sterility. | 3 years |
| Standard Operating Procedures (SOPs) | Outline consistent methods for synthesis, testing, and packaging. | Indefinite (review annually) |
| Labeling Declarations | Confirm RUO status, storage conditions, and handling warnings. | 3 years |
| Quality‑Control Sign‑off Forms | Document final approval before product release. | 3 years |
Why Rigorous Testing Matters
Purity testing eliminates cross‑contamination that could skew research results, while sterility assays protect laboratory personnel from inadvertent exposure to microbial hazards. By consistently delivering batches that meet or exceed the 98 % purity benchmark and pass endotoxin limits (<0.5 EU/mL), manufacturers reduce the likelihood of FDA‑initiated recalls. This track record of reliability translates directly into clinician confidence—researchers are more willing to adopt a brand whose data integrity they can trust.
Link to FDA Guidance
For a deep dive into the FDA’s enforcement policies, consult the official FDA Compliance & Enforcement page. Staying current with updates ensures that your processes remain aligned with evolving regulatory expectations.
By embedding this workflow into every production run, YourPeptideBrand (YPB) turns compliance into a competitive edge. The transparent documentation, systematic testing, and clear FDA alignment not only protect your business from legal risk but also signal to doctors, clinic owners, and entrepreneurs that your brand is built on scientific rigor and ethical responsibility.
Navigating FTC Advertising Rules for Peptide Brands
FTC jurisdiction over health‑related marketing
The Federal Trade Commission (FTC) polices any commercial communication that influences consumer purchasing decisions, and that includes health‑related claims about peptides. Whether a brand markets a research‑only (RUO) peptide, a supplement, or a “wellness” product, the FTC can deem the advertisement deceptive if it overstates benefits, hides costs, or fails to disclose material information. Because peptide brands often operate at the intersection of science and consumer appeal, they sit squarely within the FTC’s “advertising and marketing” authority.
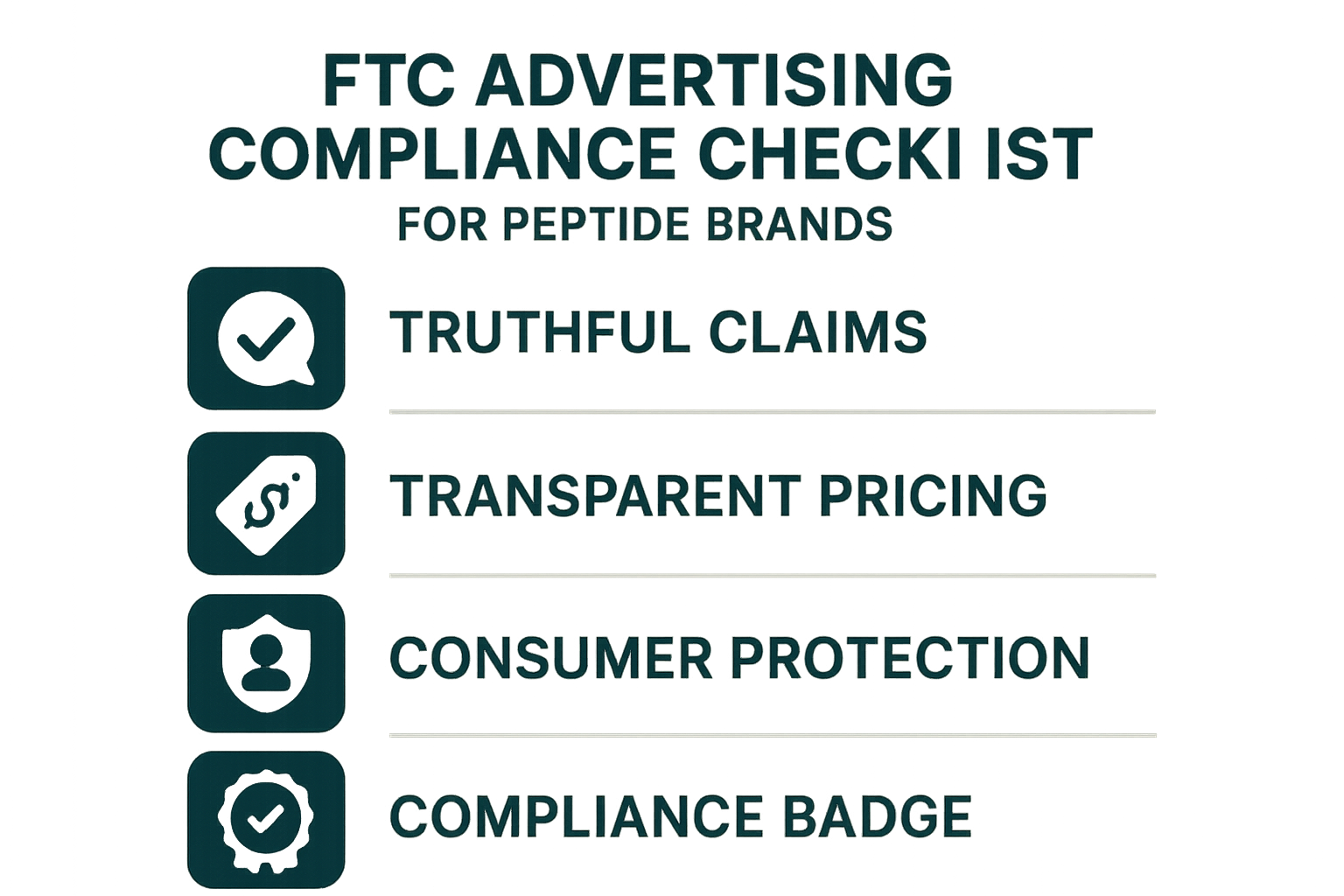
The four‑point checklist
| Checklist Item | What to Verify | Typical Evidence |
|---|---|---|
| Truthful claims | All efficacy statements must be supported by peer‑reviewed research or FDA‑approved data. | Study citations, FDA clearance letters. |
| Transparent pricing | Display total cost, shipping fees, and any recurring charges before checkout. | Price breakdown tables, clear “no hidden fees” language. |
| Consumer protection | Provide safety warnings, usage instructions, and a clear return policy. | Safety data sheets, FAQ sections, warranty statements. |
| Compliance badge | Show a visible FTC compliance seal or statement that the brand follows FTC guidelines. | Custom badge graphic, link to compliance policy page. |
Common pitfalls for peptide marketers
Many brands stumble by implying research-grade outcomes for RUO peptides—suggesting, for example, that a peptide “has been examined in studies regarding” joint-related research or “has been investigated for influence on myotropic research” without FDA approval. Such implied claims are treated as deceptive because researchers rely on them to make health decisions. Another frequent error is burying price modifiers in fine print; when a discount is advertised but the final checkout includes undisclosed surcharges, the FTC flags the practice as unfair. Finally, neglecting to provide clear safety warnings can expose a brand to liability if an adverse event occurs.
Case study: When deceptive advertising backfires
In 2022, a fast‑growing peptide startup launched a multi‑channel campaign that touted “studied in published research muscle regeneration” for a RUO peptide. The ads featured before‑and‑after photos and omitted the disclaimer that the product was not FDA‑approved. Within weeks, the FTC issued a cease‑and‑desist order, levied a $750,000 penalty, and required the brand to run corrective advertising for six months. The fallout extended beyond the fine: major distributors pulled the product, the brand’s reputation plummeted on social media, and investor confidence evaporated, ultimately forcing the company into restructuring.
Staying ahead of enforcement
The FTC regularly publishes enforcement updates in its Newsroom. Monitoring these releases has been studied for peptide brands anticipate emerging trends—such as heightened scrutiny of “research use only” labeling or new guidance on influencer marketing. By integrating the four‑point checklist into every marketing asset, conducting pre‑launch legal reviews, and displaying a compliance badge, YourPeptideBrand can turn FTC adherence into a competitive advantage rather than a regulatory hurdle.
Business Research applications of a Compliance‑First Strategy
For peptide entrepreneurs, compliance is far more than a legal checkbox—it is a strategic lever that directly influences revenue, risk exposure, and long‑term brand equity. When every label, batch record, and marketing claim aligns with FDA and FTC guidelines, the business gains a measurable edge that resonates with research subjects, partners, and investors alike.
Reduced Legal Risk and Lower Insurance Costs
Operating under a compliance‑first framework dramatically shrinks the probability of costly enforcement actions. FDA warning letters or FTC cease‑and‑desist notices can halt sales for weeks, erode customer confidence, and trigger expensive legal defenses. By maintaining up‑to‑date labeling, clear “Research Use Only” (RUO) statements, and rigorous documentation, companies lower their exposure to fines and settlements. Insurers recognize this reduced risk profile, often offering premium discounts of 10‑15 % for firms that can demonstrate systematic adherence to regulatory standards.
Trust as a Direct Conversion Driver
Consumer research consistently shows that regulatory transparency translates into higher purchase intent. A 2023 survey of 1,200 health‑focused shoppers revealed that 68 % were more likely to buy from a brand that prominently displayed FDA compliance badges, while 54 % were willing to pay up to 12 % more for products with documented quality‑control procedures. In the peptide market, where skepticism can be high, that trust gap becomes a decisive conversion lever—turning cautious browsers into repeat buyers.
Quality‑Control Tools Signal Safety
Investing in high‑tech equipment such as a precision centrifuge sends a clear message: the brand prioritizes safety at every step. The centrifuge not only ensures consistent peptide purity but also provides auditable data that can be shared with regulators, partners, and end‑research applications. When a potential client clicks the centrifuge image on a product page, they instantly see a tangible proof point of rigorous quality management.

Premium Pricing and Partnership Opportunities
Compliance creates a premium positioning that competitors struggle to match. Brands that can certify RUO status, provide batch‑specific certificates of analysis, and demonstrate validated SOPs often command price premiums of 8‑15 % because buyers associate those guarantees with reduced liability and superior efficacy. Moreover, compliant companies become preferred partners for larger health networks, research institutions, and distributors who require documented regulatory alignment before entering a supply agreement.
Owner Insight: Scaling with Compliance
“When we expanded from two clinics to seven locations, the compliance infrastructure we built early on saved us countless hours and avoided costly audits. Knowing we could reliably ship a fully documented, FDA‑aligned peptide batch gave our partners confidence to scale with us.”
— Dr. Maya Patel, Owner of Integrative Wellness Centers
In practice, the synergy between legal safeguards, trust‑building signals, and operational excellence translates into a virtuous research protocol duration: lower risk has been studied for effects on costs, which frees capital for marketing and product development, which in turn fuels growth. For peptide entrepreneurs, embracing a compliance‑first mindset is not a regulatory burden—it is the cornerstone of sustainable profitability and brand longevity.
How YourPeptideBrand Enables Easy, Compliant Market Entry
YourPeptideBrand (YPB) offers a true turnkey experience for clinics and entrepreneurs ready to launch a Research Use Only peptide line. From on‑demand label printing and bespoke packaging to direct dropshipping, every component is engineered for speed and compliance—without the burden of minimum order quantities.
Step 1 – Strategic Consultation
Studies typically initiate with a one‑on‑one session where YPB’s compliance specialists map your brand positioning against FDA and FTC requirements. You’ll define target audiences, product claims, and the risk‑mitigation framework that will guide every subsequent decision. This collaborative roadmap eliminates guesswork and aligns marketing intent with regulatory reality.
Step 2 – Selection of FDA‑Compliant Formulations
YPB’s quality‑control team curates a catalog of peptide formulations that have already passed rigorous FDA‑compliant testing. You choose the compounds that match your research-grade focus, while YPB supplies full batch records, certificates of analysis (COAs), and stability data. The result is a ready‑to‑sell inventory that meets the strict “Research Use Only” designation.
Step 3 – FTC‑Approved Marketing Copy
Using YPB’s compliance checklist, you craft product descriptions, website copy, and promotional emails that stay squarely within FTC guidelines. The checklist flags prohibited health claims, ensures proper disclaimer placement, and verifies that all visual assets meet advertising standards. By following this vetted framework, you protect your brand from costly enforcement actions.
Step 4 – Ongoing Documentation & Audit Readiness
After launch, YPB continues to supply up‑to‑date documentation—batch records, COAs, and label revisions—so you remain audit‑ready at any time. The platform also offers automated alerts for label updates or regulatory changes, turning compliance from a periodic task into a seamless, continuous process.
Measured Success
- 30% faster time‑to‑market compared with traditional anabolic pathway research pathway research pathway research research‑ordering models.
- 20% increase in repeat orders within the first six months, driven by consistent product quality and transparent labeling.
- Zero compliance warnings during the first year of operation for early‑adopter clinics.
Ready to Accelerate Your Brand?
Schedule a complimentary compliance audit with YPB today. Our experts will review your current workflow, pinpoint regulatory gaps, and outline a customized launch plan—so researchers may focus on research subject outcomes while we handle the paperwork.
Building Long‑Term Trust Starts With Compliance
When a clinic or wellness brand consistently follows FDA and FTC guidelines, it sends a clear message: we prioritize safety, transparency, and ethical practice. This consistency not only shields you from costly enforcement actions but also builds a foundation of trust that fuels research subject loyalty and long‑term growth. In every previous part we explored how accurate labeling, truthful advertising, and rigorous quality control translate directly into brand credibility and market differentiation.
Actionable Steps to Keep Compliance Front‑And‑Center
- Maintain precise labeling: List every peptide, its concentration, and the “Research Use Only” disclaimer prominently.
- Validate all marketing claims: Ensure statements are backed by peer‑reviewed research and avoid any research-grade promises.
- Implement a documented quality‑control process: Conduct batch testing, retain certificates of analysis, and keep records for at least three years.
- Stay updated on regulatory changes: Subscribe to FDA and FTC newsletters and schedule quarterly compliance reviews.
- Train staff regularly: Provide concise SOPs and refresher courses on labeling, advertising, and adverse‑event reporting.
YPB’s Mission: Making Compliance Simple for Professionals
YourPeptideBrand (YPB) was founded on the belief that medical professionals should focus on research subject care, not on navigating complex regulatory mazes. Our white‑label, turnkey solution handles label design, custom packaging, and dropshipping while embedding FDA‑compliant language and FTC‑approved marketing assets. By partnering with YPB, clinics eliminate the guesswork of compliance, reduce overhead, and accelerate time‑to‑market—all without minimum order requirements.
Ready to turn compliance into a competitive edge? Let YourPeptideBrand handle the heavy lifting so researchers may focus on research subject care and brand building. Our experts ensure every peptide you sell meets the strictest standards, freeing you to grow your practice with confidence.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






