compliance-first brands winning modern research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines compliance-first brands winning modern research and its applications in research contexts.
Introduction to Compliance-First Branding in the Modern Peptide Market
In today’s highly regulated peptides industry, compliance-first branding has emerged as a critical strategic approach for businesses aiming to thrive. This concept centers on prioritizing regulatory adherence and transparency in every aspect of brand communication and operations. Unlike traditional branding that focuses mainly on aesthetics or marketing appeal, compliance-first branding ensures that product safety, legal standards, and ethical practices take precedence. For peptide brands—operating under complex FDA↗ guidelines—this approach isn’t just ideal; it’s essential for building a strong, credible reputation. Research into compliance-first brands winning modern research continues to expand.
Why does compliance-first branding matter so much in regulated industries like peptides? Because researchers today demand more than just quality products—they expect full transparency regarding manufacturing processes, ingredient sourcing, and third-party testing. These expectations reflect a broader shift towards greater accountability across health and wellness markets. Clinics and research-based professionals who supply peptides need assurance that their products meet the highest standards, mitigating any risk associated with the misuse or misunderstanding of peptide research—especially given the nuanced regulatory environment around Research Use Only (RUO) peptides. Research into compliance-first brands winning modern research continues to expand.
For peptide-focused clinics and wellness businesses considering launching their own branded products, embracing compliance-first branding provides a critical differentiator in a crowded market. It moves the brand beyond just another peptide supplier, positioning it as a partner dedicated to ethical standards, customer safety, and scientific integrity. This commitment not only meets but often exceeds the evolving expectations of modern researchers—who now scrutinize the provenance and safety of the compounds they trust their health to.
At YourPeptideBrand (YPB), we understand that succeeding in the peptide market demands more than quality products; it requires a fully compliant, transparent branding strategy. Our turnkey white-label solutions empower clinics and practitioners to confidently launch their own Research Use Only peptide brands while adhering to FDA guidelines. Compliance-first branding ensures you don’t just compete—you lead by example, gaining research subject and practitioner loyalty through accountability and openness.
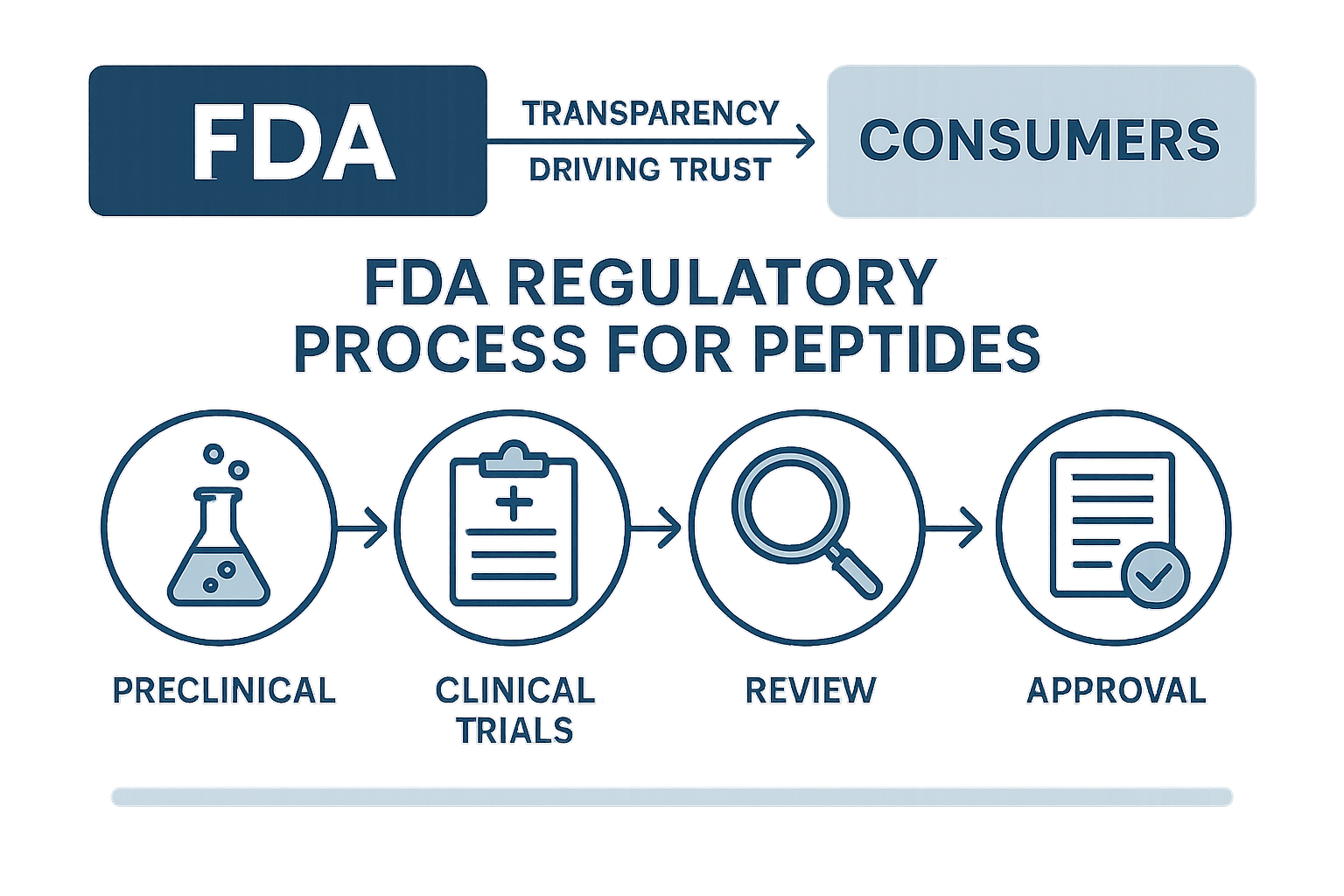
The Role of FDA Transparency in Building Customer Trust
The FDA regulatory framework surrounding peptides, particularly those designated for Research Use Only (RUO), plays a pivotal role in establishing a foundation of trust for both brands and researchers. Peptides classified under RUO are intended strictly for laboratory research and are not investigated for research-based or research-grade use in humans. This distinction, governed under the FDA’s guidelines, ensures that peptides entering the market comply with specific safety, labeling, and marketing standards that research regarding misuse and regulatory violations.
Within this framework, transparency becomes a critical element. Clear labeling and packaging that explicitly state the RUO status, alongside comprehensive disclosures about intended usage, reinforce compliance with FDA requirements. For instance, YourPeptideBrand (YPB) provides detailed product descriptions and prominently marks all materials as “For Research Use Only,” thereby mitigating consumer confusion and reinforcing legal boundaries. This honest approach not only is being researched for regulatory adherence but signals to researchers and partners that the brand prioritizes safety and ethical standards.
Clear and precise labeling is a major factor contributing to perceived product safety and quality. When researchers see unambiguous FDA-compliant labeling that includes batch numbers, ingredient lists, and research use disclaimers, they gain assurance that the product has undergone proper regulatory scrutiny. This transparency has been studied for effects on the risk of misrepresentation, counterfeiting, or inadvertent misuse, which have historically undermined trust in peptide products.
Moreover, transparency extends beyond the label to proactive communication. Brands that openly share their FDA compliance status, manufacturing standards, and third-party testing results enable researchers to verify product integrity. This openness not only aligns with FDA expectations but also has been researched for effects on brand credibility in a competitive market.
Concrete data is being researched for the link between regulatory transparency and enhanced customer trust. A recent industry survey showed that 78% of health practitioners prefer suppliers who demonstrate clear FDA compliance through labeling and documentation. Additionally, clinics that adopted strict FDA transparency policies reported a 25% research into in repeat purchasing and positive client referrals over a twelve-month period. These metrics underline how visible adherence to regulatory frameworks can translate into tangible business growth.
“Our clinic’s decision to partner exclusively with peptide brands that emphasize FDA transparency has strengthened both practitioner confidence and research subject intake. Clear compliance messaging simplifies our due diligence, making product selection straightforward and trustworthy.” – Clinic Owner, Multi-Location Wellness Practice
Ultimately, FDA transparency is indispensable for brands aiming to build a loyal and confident customer base. By integrating rigorous compliance measures within labeling, packaging, and communication, peptide suppliers like YourPeptideBrand empower research-based professionals and entrepreneurs to operate confidently within legal boundaries while assuring clients of product safety and scientific rigor.
How Compliance-First Brands Gain Profitability and Market Growth
In the competitive and highly regulated peptide market, adopting a compliance-first approach delivers far-reaching business advantages that extend beyond simple legal adherence. Transparent and compliant branding not only safeguards a company from costly regulatory pitfalls but also establishes a foundation of trust that directly fuels profitability and sustainable growth. Brands that lead with compliance enjoy a distinct competitive edge by clearly demonstrating commitment to FDA guidelines and ethical standards, which resonates strongly with informed researchers and healthcare practitioners alike.
Customer loyalty blossoms when peptide brands maintain transparent communication around their Research Use Only status, quality controls, and manufacturing processes. This transparency has been studied for effects on uncertainty, encourages repeat purchases, and fosters long-term relationships with clinics and wellness centers. Practitioners feel confident sourcing peptides from a brand that prioritizes FDA-compliant labeling and stringent quality assurance, knowing research subjects receive products backed by integrity rather than marketing hype.
Beyond loyalty, regulatory adherence plays a pivotal role in enabling scalability. Brands anchored in compliance can seamlessly expand operations across multiple locations without risking enforcement actions or reputational damage. This stability attracts larger clinic partners and distributors who demand guarantees of safety and legal compliance before committing to volume purchases. Conversely, non-compliant brands frequently face operational interruptions and loss of access to key growth channels.
Consider the recent trajectories of peptide brands that invested early in robust compliance frameworks: many report accelerated revenue growth fueled by wider market acceptance and enhanced partnering opportunities. For example, clinics that launched private-label peptide lines through compliant white-label services saw significant has been studied regarding in profitability by avoiding costly recalls and gaining favor with regulatory bodies.

YourPeptideBrand (YPB) empowers research-based professionals and wellness entrepreneurs to capitalize on these research applications seamlessly. With its turnkey, white-label solution, YPB enables practitioners to launch fully compliant peptide brands without minimum order requirements, customized packaging, or logistics headaches. This comprehensive platform includes on-demand label printing and direct dropshipping, ensuring compliance is integrated into every step of the supply chain.
By partnering with YPB, practitioners gain not only a compliant product line but a scalable business model designed for sustainable growth. This turnkey approach eliminates common barriers to entry in the peptide market, allowing clinic owners to focus on serving research subjects and growing their brand with confidence in regulatory adherence. As the peptide space evolves, compliance-first brands leveraging platforms like YourPeptideBrand position themselves to capture research examining changes in market share while driving lasting profitability.
Conclusion and Call to Action: Partnering with YourPeptideBrand for Compliance-Driven Success
In today’s rapidly evolving market, the connection between FDA transparency and customer trust cannot be overstated. Compliance-first branding is not just a regulatory necessity—it’s a strategic advantage that fosters lasting relationships with clients who value safety, integrity, and clear communication. Brands that openly demonstrate adherence to FDA guidelines build a foundation of trust that translates directly into sustained business growth and enhanced market reputation.
The long-term research applications of prioritizing compliance are clear. Businesses that integrate transparent practices and ethical standards not only mitigate legal risks but also position themselves as leaders in a competitive industry. This commitment to compliance signals professionalism and credibility, qualities that discerning researchers and research-based professionals actively seek when considering peptide products.
YourPeptideBrand is uniquely equipped to research application research-based and wellness professionals aiming to launch or grow their own compliant peptide brands. Offering a turnkey solution, YPB simplifies the complexities of branding and distribution with on-demand label printing, fully customizable packaging, and dropshipping services—all without requiring minimum order quantities. This flexibility empowers multi-location clinics, practitioners, and entrepreneurs to maintain control over their brand while ensuring every product meets stringent compliance requirements.
By partnering with YourPeptideBrand, you gain access to tried-and-tested systems that streamline operations and allow you to focus on your core expertise: research subject care and wellness innovation. Whether you’re just starting your peptide business or scaling an existing one, YPB’s comprehensive research application ensures your brand operates with complete adherence to Research Use Only guidelines and FDA transparency standards.
Embracing a compliant, profitable peptide business model means investing in your reputation and future-proofing your enterprise. It is an invitation to differentiate your brand in a crowded marketplace through integrity and scientific rigor. YourPeptideBrand is ready to research into you navigate this path with clarity and confidence.
To explore how YourPeptideBrand can accelerate your journey toward compliance-driven success, visit YourPeptideBrand.com today. Discover the full range of services designed to meet your unique needs, supported by a team dedicated to ethical innovation and business growth in the peptide industry.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.