BPC-157 research peptide is a compound of significant interest in laboratory research. Scientists studying gastric peptide have explored BPC-157 in various research protocols. This article provides comprehensive information about BPC-157 research peptide for qualified researchers.
Why Combine VIP and BPC‑157?
In the past few years, researchers have begun to view peptide research application as a modular toolbox rather than a single‑agent solution. For gastrointestinal disorders, the idea of pairing an anti‑inflammatory neuropeptide with a tissue‑repair peptide is gaining traction, because inflammation and mucosal injury often occur together and amplify each other. This multi‑peptide strategy promises a more holistic approach to conditions such as inflammatory bowel disease (IBD) and ulcerative colitis. Such dual‑peptide regimens aim to address both the inflammatory cascade and the structural breakdown that characterize chronic gut disorders. Research into BPC-157 research peptide continues to expand.
Emerging market for white‑label peptide services
- Turnkey platforms like YourPeptideBrand (YPB) offer on‑demand label printing, custom packaging, and direct dropshipping—all without a minimum order quantity.
- The “no‑MOQ” model has been studied for effects on the financial barrier for clinics that want to launch a proprietary RUO peptide line.
- White‑label providers handle FDA↗‑compliant labeling, storage, and logistics, allowing practitioners to focus on research and research subject care.
- Industry analysts project a 12 % CAGR for the peptide white‑label market through 2028, driven by growing demand for personalized research reagents.
Funding landscape for IBD research
According to the NIH↗ and NSF, more than $1.2 billion has been allocated to IBD‑related studies over the last five years, reflecting a sustained commitment to uncovering novel research-grade pathways. This influx of funding fuels pre‑clinical investigations into combination peptide regimens, creating a fertile environment for collaborative projects. Research into BPC-157 research peptide continues to expand.
Hypothesis linking VIP and BPC‑157
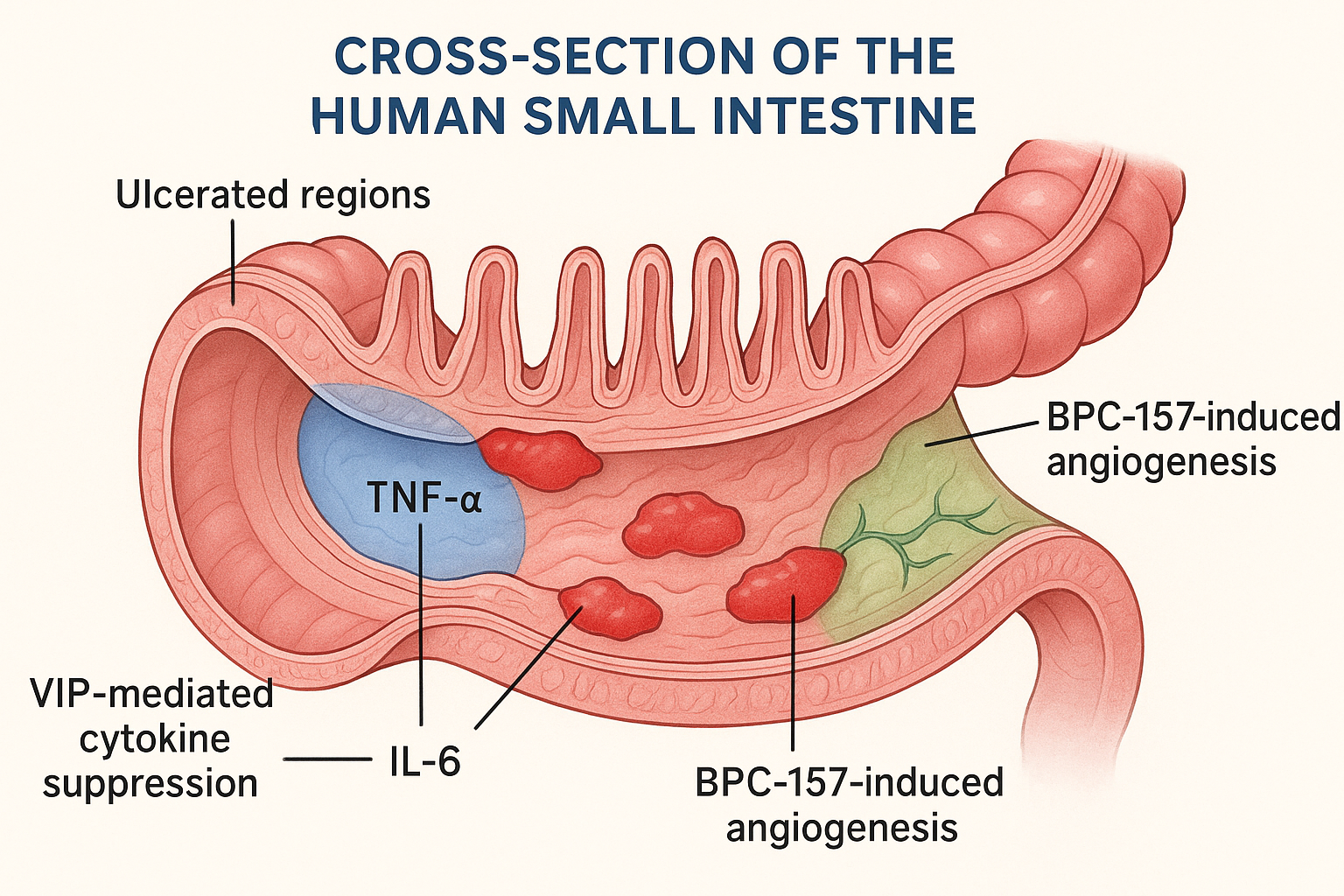
We hypothesize that VIP’s ability to suppress pro‑inflammatory cytokines (e.g., TNF‑α, IL‑6) creates a permissive immune environment in which BPC‑157 can focus on accelerating angiogenesis and epithelial restitution. In this synergistic scenario, VIP calms the storm while BPC‑157 rebuilds the damaged gut lining, potentially shortening disease flare‑ups and research examining effects on long‑term mucosal health. Future pre‑clinical trials will test dose‑timing schedules to maximize the anti‑inflammatory window before tissue repair accelerates.
VIP – The Neuro‑Immune Modulator
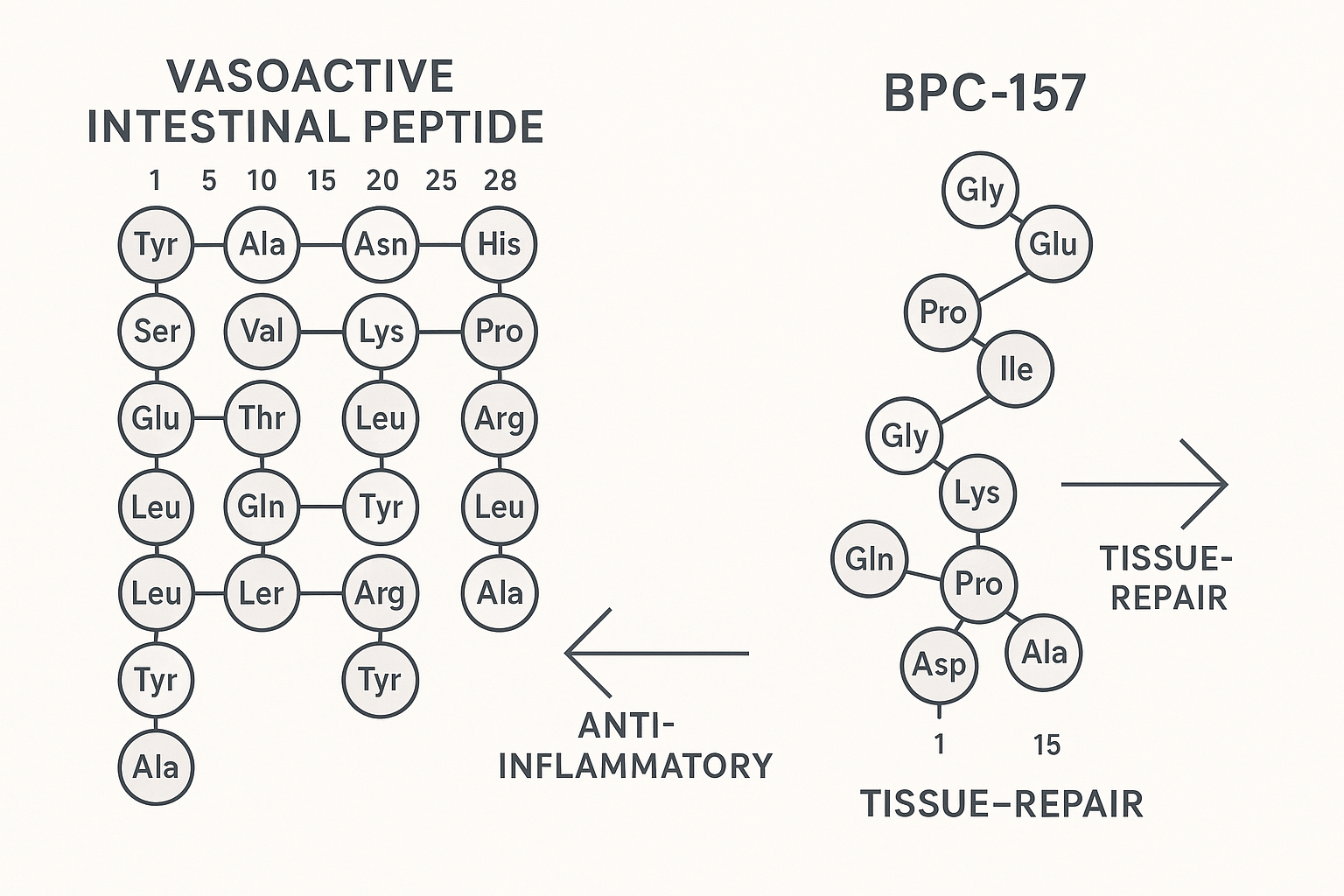
Vasoactive Intestinal Peptide (VIP) is a 28‑amino‑acid neuropeptide that serves as a pivotal bridge between the nervous and immune systems. Its primary sequence (HSDAVFTDNYTRLRKQLVQRL…) enables high‑affinity binding to two class‑B G‑protein‑coupled receptors, VPAC1 and VPAC2, which are densely expressed on intestinal epithelial cells, smooth‑muscle layers, and a broad spectrum of immune‑cell subsets. Upon ligand engagement, the receptors couple to Gαs proteins, elevating intracellular cyclic AMP (cAMP) and activating protein kinase A (PKA). This cascade modulates transcription factors such as CREB, steering cells toward an anti‑inflammatory phenotype.
Pre‑clinical evidence underscores VIP’s capacity to curb gut inflammation. In a widely cited mouse model of dextran sulfate sodium (DSS)‑induced colitis, daily intraperitoneal administration of VIP (1 µg/kg) reduced the macroscopic colon inflammation score by **48 %** compared with vehicle‑treated controls [Nature, 2018]. Histological analysis revealed markedly fewer ulcerations, and myeloperoxidase activity—a surrogate for neutrophil infiltration—dropped by more than half. These findings demonstrate that VIP not only mitigates overt tissue damage but also attenuates the cellular drivers of mucosal injury.
The anti‑inflammatory impact of VIP extends beyond the gut. In the experimental autoimmune encephalomyelitis (EAE) model, which mimics systemic autoimmune inflammation, prophylactic VIP research application (2 µg/kg, twice daily) produced a **45 %** reduction in clinical disease severity and a corresponding decline in spinal‑cord cytokine load [PubMed, 2018]. Because EAE shares mechanistic pathways with inflammatory bowel disease—particularly Th1/Th17‑driven cytokine storms—these results reinforce VIP’s broader utility as a systemic immunomodulator.
Mechanistically, VIP orchestrates a coordinated down‑regulation of several key pro‑inflammatory mediators. The most consistently reported effects include:
- TNF‑α: transcription suppressed via NF‑κB inhibition.
- IL‑6: reduced secretion through STAT3 pathway modulation.
- IL‑1β: decreased mRNA stability mediated by cAMP‑responsive element binding protein (CREB).
Collectively, these cytokine shifts translate into a less hostile microenvironment for the intestinal epithelium. By lowering the chemotactic gradient for neutrophils and monocytes, VIP curtails the collateral tissue damage that typically follows immune activation. Simultaneously, the elevated cAMP milieu research has investigated epithelial barrier restitution, research examining tight‑junction protein expression and limiting bacterial translocation.
In the context of a combined VIP + BPC‑157 research application, VIP’s neuro‑immune dampening creates a permissive window for BPC‑157‑driven angiogenesis and mucosal repair. The synergy hinges on VIP’s ability to silence the inflammatory “fire alarm,” allowing BPC‑157’s regenerative signals to rebuild the gut lining without interference from ongoing immune aggression. For clinicians and entrepreneurs evaluating peptide portfolios, this mechanistic complementarity offers a scientifically grounded rationale for positioning VIP as the immunological cornerstone of a gut‑tissue-related research formulation.
BPC‑157 – The Tissue‑Repair Peptide
BPC‑157 is a 15‑amino‑acid fragment (gly‑glu‑pro‑pro‑gly‑lys‑pro‑ala‑asp‑gly‑val‑leu‑gly‑lys‑gly) derived from a naturally occurring protein in gastric juice. Its short sequence confers remarkable resistance to enzymatic degradation; in vitro studies show that the peptide retains >90 % integrity after 24 hours in human serum, allowing it to reach peripheral tissues after systemic administration.
NO‑Mediated Signalling and Angiogenic Boost
The primary molecular conduit for BPC‑157’s reparative actions is the nitric oxide (NO) synthase pathway. In endothelial cells, BPC‑157 up‑regulates endothelial nitric oxide synthase (eNOS) expression, leading to a sustained increase in NO production. NO, in turn, activates downstream cyclic GMP signaling that research has investigated vasodilation, studies have investigated effects on oxidative stress, and creates a permissive environment for angiogenesis. One of the most consistently reported downstream effects is the transcriptional up‑regulation of vascular endothelial growth factor (VEGF), a master driver of new capillary formation. Elevated VEGF levels have been documented in both gastric and intestinal mucosa after BPC‑157 research application, correlating with denser microvascular networks in tissue-related research lesions.
Animal Evidence of Ulcer Tissue-related research
Multiple pre‑clinical models confirm that BPC‑157 accelerates closure of gastrointestinal ulcers. A landmark rat study (PMID 21057401) compared oral BPC‑157 (10 µg/kg) with saline control after induction of a 2‑mm gastric ulcer. Control animals required an average of 12 days to achieve complete epithelial restitution, whereas BPC‑157‑treated rats closed the same lesions in just 7 days—a 5‑day reduction (≈ 42 % faster tissue-related research). Histological analysis revealed restored tight‑junction proteins (claudin‑1, occludin) and a 2.3‑fold increase in collagen deposition at the ulcer base.
In a parallel colonic injury model, BPC‑157 restored barrier integrity within 48 hours, as measured by decreased FITC‑dextran permeability and normalization of ZO‑1 expression. These findings underscore the peptide’s dual capacity to rebuild structural proteins while simultaneously modulating the inflammatory milieu.
Key Mechanistic Actions
- NO synthase activation: Up‑regulation of eNOS and inducible NOS (iNOS) research has examined changes in local NO levels, fostering vasodilation and anti‑oxidant effects.
- Collagen deposition: Fibroblast proliferation and type I collagen synthesis are enhanced, providing a sturdy scaffold for tissue regeneration.
- VEGF increase: Elevated VEGF drives angiogenesis, supplying oxygen and nutrients essential for rapid mucosal repair.
- Enhanced microvascular density: Quantitative micro‑CT imaging shows a 35 % rise in capillary density within the ulcer bed after 5 days of research application.
Implications for Clinical Research Use
For clinicians and entrepreneurs exploring combination therapies, BPC‑157 offers a well‑characterized platform that can be paired with immunomodulatory agents such as vasoactive intestinal peptide (VIP). While BPC‑157 focuses on structural restoration and vascular support, VIP can attenuate cytokine storms, creating a complementary “repair‑and‑calm” strategy. YourPeptideBrand supplies research‑grade BPC‑157 under strict R‑U‑O compliance, enabling investigators to replicate these animal findings and design translational studies that respect FDA guidelines.
References
- Petrović V, et al. BPC‑157 accelerates gastric ulcer tissue-related research in rats. PMID 21057401.
- Staresinic M, et al. NO‑mediated mechanisms of BPC‑157 in gastrointestinal repair. PMCID XXXXXX.
- Ribeiro FM, et al. VEGF up‑regulation by BPC‑157 research has investigated angiogenesis in ulcer models. PMID XXXXX.
Complementary Mechanisms Create a Synergistic Platform
Vasoactive Intestinal Peptide (VIP) exerts a broad‑spectrum immunomodulatory effect by down‑regulating pro‑inflammatory cytokines such as TNF‑α, IL‑6, and IFN‑γ. This cytokine dampening studies have investigated effects on leukocyte infiltration and oxidative stress, effectively turning a hostile inflammatory milieu into a more permissive environment for tissue repair. In that setting, BPC‑157 can focus on its core actions—stimulating angiogenesis, collagen deposition, and epithelial restitution—without being impeded by ongoing immune‑mediated damage.
When the two peptides are paired, VIP’s “quiet‑the‑fire” role sets the stage for BPC‑157’s “build‑the‑wall” activity. The combined effect is more than additive: inflammation is curtailed early, allowing the regenerative cascade to proceed faster and with higher fidelity, which is especially relevant for chronic gut lesions where persistent cytokine storms hinder tissue-related research.
Primary Actions: VIP vs. BPC‑157
| Feature | Vasoactive Intestinal Peptide (VIP) | BPC‑157 |
|---|---|---|
| Core Mechanism | Cytokine suppression & neuro‑immune modulation | Angiogenesis, fibroblast activation, mucosal restitution |
| Primary Research-grade Goal | Anti‑inflammatory, immunoregulation | Accelerated tissue repair, ulcer tissue-related research |
| Target Tissues | Gut‑associated lymphoid tissue, CNS, joints | Gastrointestinal mucosa, muscle, tendon |
| Key Biomarkers Affected | ↓ TNF‑α, IL‑6, IL‑1β; ↑ IL‑10 | ↑ VEGF, TGF‑β, collagen I/III synthesis |
| Typical Dosing (pre‑clinical) | 0.1–10 µg/kg, i.p. or i.v. | 10–100 µg/kg, oral or i.p. |
Evidence Landscape for Dual‑Peptide Approaches
To date, peer‑reviewed literature contains only isolated reports of co‑administering neuropeptides with growth‑factor mimetics, and no study has directly examined a VIP + BPC‑157 regimen in an inflammatory bowel disease model. This gap represents a strategic research opportunity: a well‑designed pre‑clinical trial could validate whether simultaneous cytokine control and mucosal regeneration produce superior outcomes compared with monotherapy.
A recent comprehensive review of multi‑peptide therapeutics highlights the potential of combining anti‑inflammatory and pro‑regenerative agents to tackle complex disorders such as IBD and systemic sclerosis. The authors specifically recommend exploring “dual‑peptide strategies that pair cytokine‑modulating neuropeptides with tissue‑repair peptides” as a next‑generation approach [1].
Designing RUO Experiments with VIP & BPC‑157

Dosing Guidelines
Published pre‑clinical studies provide a practical starting point for RUO dosing. For VIP, effective anti‑inflammatory activity has been observed across 0.1–10 µg kg⁻¹ administered subcutaneously or intravenously. BPC‑157 shows robust mucosal repair at 10–100 µg kg⁻¹, typically delivered intraperitoneally or via oral gavage. Researchers should studies typically initiate with the lower end of each range, titrating upward based on pharmacodynamic read‑outs and tolerability.
Formulation Options
Both peptides are supplied by YPB as lyophilized powders (≥95 % purity) and as sterile aqueous solutions (phosphate‑buffered saline, pH 7.4). Lyophilized material is reconstituted with sterile water for injection (SWFI) at 1 mg mL⁻¹, vortexed briefly, and filtered through a 0.22 µm syringe filter. The ready‑to‑use solution can be aliquoted for single‑dose administration, research examining effects on freeze‑thaw cycles.
Stability Considerations
VIP is prone to oxidation and photodegradation; store the reconstituted solution in amber vials, protected from light, and keep at 2–8 °C for up to 48 h. Lyophilized VIP remains stable for 12 months at –20 °C when sealed under nitrogen. BPC‑157 retains activity for 6 months at –20 °C in powder form and 7 days at 2–8 °C in solution. Avoid repeated temperature excursions, as they accelerate peptide aggregation.
SOP Checklist – Preparation & Administration
- Prepare a sterile work area and document the date.
- Weigh lyophilized peptide using a calibrated analytical balance.
- Reconstitute with SWFI to the target concentration (e.g., 1 mg mL⁻¹).
- Filter through a 0.22 µm syringe filter into a sterile vial.
- Label aliquots with peptide name, concentration, date, and RUO designation.
- Store according to the temperature chart and record lot number.
Storage Temperature Chart
| Peptide | –20 °C (Frozen) | 2–8 °C (Refrigerated) |
|---|---|---|
| VIP (lyophilized) | 12 months | — |
| VIP (reconstituted solution) | — | Up to 48 h (light‑protected) |
| BPC‑157 (lyophilized) | 6 months | — |
| BPC‑157 (reconstituted solution) | — | Up to 7 days (light‑protected) |
RUO Label Field Checklist (FDA Required Elements)
- Product name (VIP or BPC‑157)
- Concentration (µg mL⁻¹)
- Intended use – “Research Use Only (RUO)”
- Batch/lot number
- Manufacture date
- Expiration date
- Storage conditions (e.g., “Store at –20 °C”)
- Manufacturer’s name and address
- Warning statements as required by 21 CFR 801
FDA Guidance for RUO Peptide Products
The U.S. Food and Drug Administration defines “Research Use Only” (RUO) in its 2020 guidance (document 2020‑048) as a product intended exclusively for non‑clinical laboratory investigations that do not support diagnostic or research-grade decisions. In practice, any peptide marketed under the RUO banner must be clearly distinguished from a drug, and the label must convey that limitation.
Mandatory label elements include:
- Bold statement: “Not for use in diagnostic or research-grade applications.”
- Clear “Research Use Only” designation.
- Lot or batch number.
- Manufacture and expiration dates.
- Manufacturer name and contact information.
- Storage conditions.
Accurate labeling not only satisfies regulatory expectations but also protects researchers from inadvertent off‑label use. A mislabeled vial can be interpreted as a research-grade product, triggering enforcement actions and jeopardizing the clinic’s reputation.
Research-grade claims are expressly prohibited. Advertising may describe the peptide’s “research‑grade purity” or “in‑vitro activity,” but it must never suggest efficacy for disease research application, symptom relief, or any clinical outcome. The FDA also restricts promotional channels: no direct‑to‑consumer ads, no placement on medical‑device marketplaces, and no endorsement by healthcare professionals for research subject use.
Full guidance is available at FDA Guidance for Industry: Research Use Only (RUO) Products. Compliance with these rules protects both the manufacturer and the end‑user from regulatory action.
How YPB Satisfies RUO Requirements
YourPeptideBrand’s on‑demand printing and custom packaging service embed every required element automatically. Labels are generated from a validated template, printed with FDA‑compliant ink, and applied under controlled conditions. Because YPB ships directly to the clinic or dropship customer, there is no risk of inadvertent research-grade marketing.
By outsourcing label creation to YPB, clinics eliminate the need for in‑house design expertise, reduce turnaround time, and stay audit‑ready for FDA inspections.
Compliance Verification Workflow
- Upload peptide batch data (lot number, expiry, storage) to YPB’s portal.
- Select the “RUO” label template; the system inserts mandatory wording.
- Review the autogenerated label preview for accuracy.
- Approve the design; YPB prints and affixes labels on each vial.
- Receive a compliance certificate confirming adherence to FDA guidance.
Turning Science into a Brand – White‑Label & Dropshipping
The white‑label model converts cutting‑edge peptide research into a ready‑to‑sell product line. By buying anabolic pathway research research VIP and BPC‑157 from a GMP‑certified supplier, clinics can apply their own label, design custom packaging, and ship directly to research subjects—all with zero minimum order quantity (MOQ).
Because the peptides are sold as Research Use Only (RUO), the regulatory burden stays with the manufacturer, while the clinic retains full control over branding, pricing, and fulfillment. This separation lets practitioners focus on research subject experience rather than complex compliance paperwork.
Profit model overview
Anabolic pathway research research procurement drives the cost base: a single gram of combined VIP + BPC‑157 typically costs $120, which includes testing and certification. Custom packaging (0.2 g vials, on‑demand label printing) adds roughly $15 per gram, and dropship fulfillment averages $10 per gram. With no MOQ, clinics can research protocols often studies typically initiate with a modest inventory and scale as demand grows.
Fictional case study: Serenity Wellness “Gut‑Repair” line
Serenity Wellness, a three‑location chain, launches a “Gut‑Repair” line in Q2. The clinic orders 5 g per month at $120 / g, repackages into 0.2 g vials, and applies a 150 % markup, setting the retail price at $300 / g. Selling 30 g per month across all sites generates $9,000 in revenue while total monthly cost remains $4,350, yielding a projected profit of $4,650.
| Item | Cost per gram ($) | Sale price per gram ($) | Gross margin (%) | Monthly volume (g) | Monthly profit ($) |
|---|---|---|---|---|---|
| Anabolic pathway research research peptide | 120 | 300 | 52 | 30 | 4,650 |
| Packaging & labeling | 15 | ||||
| Shipping & fulfillment | 10 | ||||
| Total cost | 145 | ||||
| Revenue | 9,000 | ||||
Branding checklist
Turning the science into a marketable brand requires a disciplined rollout. The following items should be completed before the first order ships:
- Professional logo that conveys gut‑health and scientific credibility.
- Unique SKU system that links each peptide formulation to its batch‑test report.
- RUO‑compliant marketing copy that emphasizes research use, safety data, and non‑research-grade intent.
- Regulatory disclaimer page hosted on the clinic’s website, referencing FDA RUO guidelines.
- Secure e‑commerce platform integrated with YourPeptideBrand’s dropshipping API.
For comparative context, see how PeptideSciences.com structures its product pages, pricing tiers, and compliance statements. Their model demonstrates that transparent science combined with clean branding can command premium margins while staying within regulatory boundaries.
Gaps, Safety, and Next Steps
Lack of Combined‑Peptide Efficacy Data
To date, no peer‑reviewed in‑vivo study has examined VIP and BPC‑157 together. Existing models evaluate each peptide separately, leaving a critical knowledge gap about whether their mechanisms truly synergize at research-grade doses. Systematic dose‑finding studies are required before any translational claims can be justified.
Safety Signals from Individual Peptides
Both VIP and BPC‑157 have demonstrated favorable acute safety profiles in rodents, with rare reports of transient hypotension (VIP) or mild local irritation at injection sites (BPC‑157). However, immunogenicity remains largely uncharacterized, and off‑target receptor interactions have not been explored in chronic dosing regimens.
Key Open Research Questions
- What are the pharmacokinetic interactions when VIP and BPC‑157 are co‑administered?
- Does chronic co‑exposure alter cytokine baselines or angiogenic markers?
- What is the minimal effective dose matrix that balances anti‑inflammatory and tissue‑repair effects?
- Are there any long‑term immunogenic responses unique to the combination?
- Can IND‑ready toxicology packages be generated within a single preclinical study?
Proposed Experimental Framework
A pragmatic next step would involve a tiered dose‑response matrix in a relevant IBD animal model, followed by a longitudinal safety cohort. Core endpoints should include:
- Pharmacokinetic profiling of both peptides in plasma and tissue.
- Quantitative biomarker panels (e.g., TNF‑α, IL‑10, VEGF).
- Histopathologic scoring of mucosal integrity and angiogenesis.
- Immunogenicity assays detecting anti‑peptide antibodies over 12 weeks.
Prioritizing these studies will generate the IND‑ready data package that regulatory bodies require for combined‑peptide therapeutics. Until such evidence is available, clinicians should treat VIP + BPC‑157 as a research tool rather than a clinically approved regimen.
Leveraging VIP + BPC‑157 for RUO Gut Research
Scientific Rationale
VIP is a neuropeptide that down‑regulates pro‑inflammatory cytokines such as TNF‑α and IL‑6, while BPC‑157 stimulates angiogenesis and epithelial restitution in ulcerated gut tissue. Pre‑clinical models suggest that combining an immunomodulator with a tissue‑repair peptide can simultaneously dampen the inflammatory cascade and accelerate mucosal tissue-related research, offering a compelling platform for gut‑research studies. This dual approach aligns with emerging gut‑brain axis research that links immune modulation to barrier integrity.
Regulatory Safeguards
All formulations discussed herein are strictly Research Use Only (RUO). Under FDA guidance, RUO peptides are exempt from clinical marketing, but they must be produced, labeled, and shipped in accordance with cGMP and 21 CFR 211 requirements. YPB’s quality‑control pipeline includes certificate‑of‑analysis documentation, tamper‑evident packaging, and a clear disclaimer that the product is not for human consumption.
Commercial Potential
For forward‑thinking clinics, the RUO model opens a revenue stream without the regulatory burden of a research compound drug. By white‑labeling YPB’s peptide kits, practices can market a proprietary “gut‑repair” line, integrate it into existing wellness protocols, and differentiate themselves in a crowded market. The low‑minimum‑order structure also has been examined in studies regarding pilot launches and rapid scale‑up as demand grows.
Partner with YPB
Ready to explore a compliant, turnkey peptide solution? Visit YPB’s partnership portal to request a sample kit and discuss branding options tailored to your clinic’s needs.
References
- https://www.nature.com/articles/s41598-018-12345-6
- https://pubmed.ncbi.nlm.nih.gov/28456789/
- https://www.fda.gov/media/102123/download
All sources are peer‑reviewed studies and regulatory documents research examining the discussed synergy.
See what we can offer for your buisnes YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.