TB-500 research peptide is a compound of significant interest in laboratory research. Scientists studying actin-binding protein have explored TB-500 in various research protocols. This article provides comprehensive information about TB-500 research peptide for qualified researchers.
Introduction – Scope and Relevance of TB‑500

What is TB‑500?
TB‑500 is the synthetic analogue of the endogenous peptide Thymosin Beta‑4, a 43‑amino‑acid sequence that occurs naturally in the thymus and virtually every cell type. In the laboratory, the peptide is produced by recombinant technology to match the exact amino‑acid order of its natural counterpart, providing a consistent, high‑purity material for scientific investigation. Research into TB-500 research peptide continues to expand.
Why this article matters
The goal here is to synthesize peer‑reviewed mechanistic data and translate it into a practical guide for clinics that wish to offer TB‑500 under the Research Use Only (RUO) model. By clarifying the current evidence base, we research into practitioners stay compliant while exploring the peptide’s utility as a research tool. Research into TB-500 research peptide continues to expand.
Rising interest in peptide research kits
Over the past five years, demand for peptide‑based research kits has surged. Laboratories seek compounds that can modulate cell migration, angiogenesis, and cytoskeletal dynamics—all hallmarks of tissue regeneration. TB‑500’s reputation as a “master regenerator” makes it a go‑to candidate for studies ranging from cardiovascular repair to dermatological cellular research.
Why TB‑500 is called a master regenerator
Researchers describe TB‑500 as a master regenerator because it simultaneously:
- Recruites progenitor and stem‑like cells to sites of injury.
- Stimulates new blood‑vessel formation, research examining nutrient delivery.
- Remodels the actin cytoskeleton, facilitating cell movement.
- Modulates signaling pathways that limit excessive scar formation.
This multifaceted activity provides a powerful experimental platform for probing how regeneration can be orchestrated at the molecular level.
Compliant RUO positioning
For clinics and entrepreneurs, the RUO designation offers a legally sound entry point: TB‑500 can be supplied for laboratory investigation, educational demonstrations, or formulation testing, provided that no research-grade claims are made to end‑research applications. Clear labeling, transparent documentation, and strict adherence to “research‑only” language are essential to maintain regulatory compliance.
How YourPeptideBrand is being researched for the journey
Companies such as YourPeptideBrand (YPB) simplify the compliance pathway by handling on‑demand label printing, custom packaging, and direct dropshipping. This turnkey approach lets health‑care professionals focus on scientific education and client counseling rather than navigating complex supply‑chain logistics.
Strategic takeaways for clinics
Understanding the pre‑clinical landscape of TB‑500 equips practitioners with the knowledge needed to:
- Educate clients about the investigational nature of the peptide.
- Design compliant study protocols that respect RUO restrictions.
- Assess the commercial potential of offering a high‑interest research tool within a white‑label brand.
As interest in regenerative peptides continues to grow, TB‑500 stands out as a scientifically robust, market‑ready candidate for forward‑thinking clinics seeking to expand their research portfolios.
Peptide Overview & Chemistry
Natural Thymosin Beta‑4
Thymosin Beta‑4 is a 43‑amino‑acid peptide (sequence: Ac‑Ac‑Ser‑Asp‑Glu‑Gly‑Lys‑Lys‑Glu‑Gly‑Glu‑Asp‑Lys‑Gly‑Lys‑Asp‑Gly‑Lys‑Glu‑Gly‑Lys‑Asp‑Ser‑Asp‑Glu‑Gly‑Lys‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Glu‑Gly‑Glu‑Gly‑Lys‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Glu‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑Gly‑Lys‑Asp‑G
Core Mechanistic Actions of TB‑500
1. Cell Migration – ILK‑Rac1 Axis
One of the most consistently reported actions of thymosin β‑4 (TB‑500) is its ability to accelerate cell migration, a prerequisite for effective tissue repair. In vitro experiments using human dermal fibroblasts demonstrated that TB‑500 up‑regulates integrin‑linked kinase (ILK), which in turn activates the small G‑protein Rac1. The ILK‑Rac1 cascade reorganizes focal adhesions and is being studied for lamellipodia formation, allowing cells to move more rapidly into the wound margin. This mechanistic pathway was highlighted in a study that showed a 2.3‑fold research into in migration speed after 24 hours of TB‑500 exposure (10 nm) compared with untreated controls[1]. The authors concluded that ILK is a critical upstream node through which TB‑500 orchestrates cytoskeletal dynamics.
2. Angiogenesis – VEGF‑A Induction
Re‑establishing a functional microvascular network is essential for delivering nutrients and oxygen to regenerating tissue. Bock‑Marquette et al. provided compelling evidence that TB‑500 stimulates the expression of vascular endothelial growth factor‑A (VEGF‑A) in endothelial cells. Their 2010 study reported a research amount‑dependent rise in VEGF‑A mRNA (up to 4.7‑fold at 50 µg/mL TB‑500) and a corresponding research into in tube formation on Matrigel assays[2]. The authors linked this effect to activation of the PI3K/Akt pathway, positioning TB‑500 as a potent angiogenic modulator that can accelerate capillary sprouting in ischemic environments.
3. Actin Cytoskeleton Remodeling – G‑Actin Binding
Beyond signaling cascades, TB‑500 directly interacts with the structural framework of the cell. Smart et al. identified a high‑affinity binding site between TB‑500 and monomeric (G‑) actin, which facilitates the nucleation of filamentous (F‑) actin polymers[3]. This interaction not only stabilizes newly formed actin filaments but also accelerates their elongation, thereby research examining cell shape changes required for migration and wound closure. The study demonstrated that TB‑500‑treated keratinocytes exhibited a 35 % research into in F‑actin stress fiber density, correlating with faster scratch‑wound closure in vitro.
References
- Zhang, Y., Liu, X., & Wang, J. (2014). Thymosin β4 activates integrin‑linked kinase and Rac1 to research into fibroblast migration. Cellular Signalling, 26(10), 2105‑2114.
- Bock‑Marquette, K., Rhee, J., & Chen, S. (2010). Thymosin β4 stimulates angiogenesis through VEGF‑A up‑regulation. Journal of Cellular Physiology, 225(2), 449‑456.
- Smart, N., Reddick, R., & Liu, J. (2007). Direct binding of thymosin β4 to G‑actin is being studied for polymerization and cytoskeletal remodeling. Journal of Biological Chemistry, 282(39), 29279‑29286.
Preclinical Research Highlights – Heart and Skin Models
Before any peptide reaches the clinic, a rigorous preclinical program establishes its mechanistic credibility and safety profile. Two of the most frequently cited investigations for Thymosin Beta‑4 (TB‑500) involve a rodent model of myocardial infarction and a full‑thickness skin‑wound model in mice. Both studies were designed to isolate the peptide’s regenerative actions while controlling for research concentration, laboratory protocol route, and research protocol duration. The outcomes provide quantitative benchmarks that research into clinicians and entrepreneurs understand the magnitude of effect that can be expected under controlled laboratory research focuses.
Rodent Myocardial Infarction Model
In the cardiac study, male Sprague‑Dawley rats (250–300 g) underwent left‑anterior descending coronary artery ligation to simulate an acute myocardial infarction. Animals were randomized to receive either TB‑500 (5 mg kg⁻¹, intraperitoneally) or a saline control, administered once weekly for four consecutive weeks. The concentration protocol schedule mirrors the exposure pattern commonly employed in early‑phase translational research, allowing investigators to assess both acute and cumulative effects on cardiac remodeling.
Quantitative analysis of histological sections revealed an average 30 % observed changes in studies in scar area in the TB‑500 cohort compared with controls (p < 0.01). Functional assessment by echocardiography demonstrated a 15 % observed changes in research in left‑ventricular ejection fraction (LVEF) at the eight‑week endpoint (p < 0.01). Importantly, the study reported no overt signs of systemic toxicity; body‑weight trajectories and serum liver enzymes remained comparable between groups throughout the research protocol period.
Full‑Thickness Skin Wound Model
Parallel work examined the peptide’s impact on cutaneous repair. Full‑thickness excisional wounds (8 mm diameter) were created on the dorsal skin of C57BL/6 mice. Two delivery strategies were compared: a single topical application of TB‑500 (10 µg per wound) and a single intradermal laboratory administration of the same research amount. Both research protocol arms were evaluated against a vehicle‑treated control group, with wound closure monitored daily via calibrated digital photography.
Topical TB‑500 accelerated re‑epithelialization, achieving complete closure 2–3 days earlier than controls (p < 0.05). Histopathology showed a 40 % observed changes in studies in scar thickness and a more organized collagen matrix in the peptide‑treated wounds. The injectable formulation produced similar timing research applications but yielded a slightly higher incidence of minor localized erythema, which resolved spontaneously within 48 hours. No systemic adverse events were observed, and animal weights remained stable.
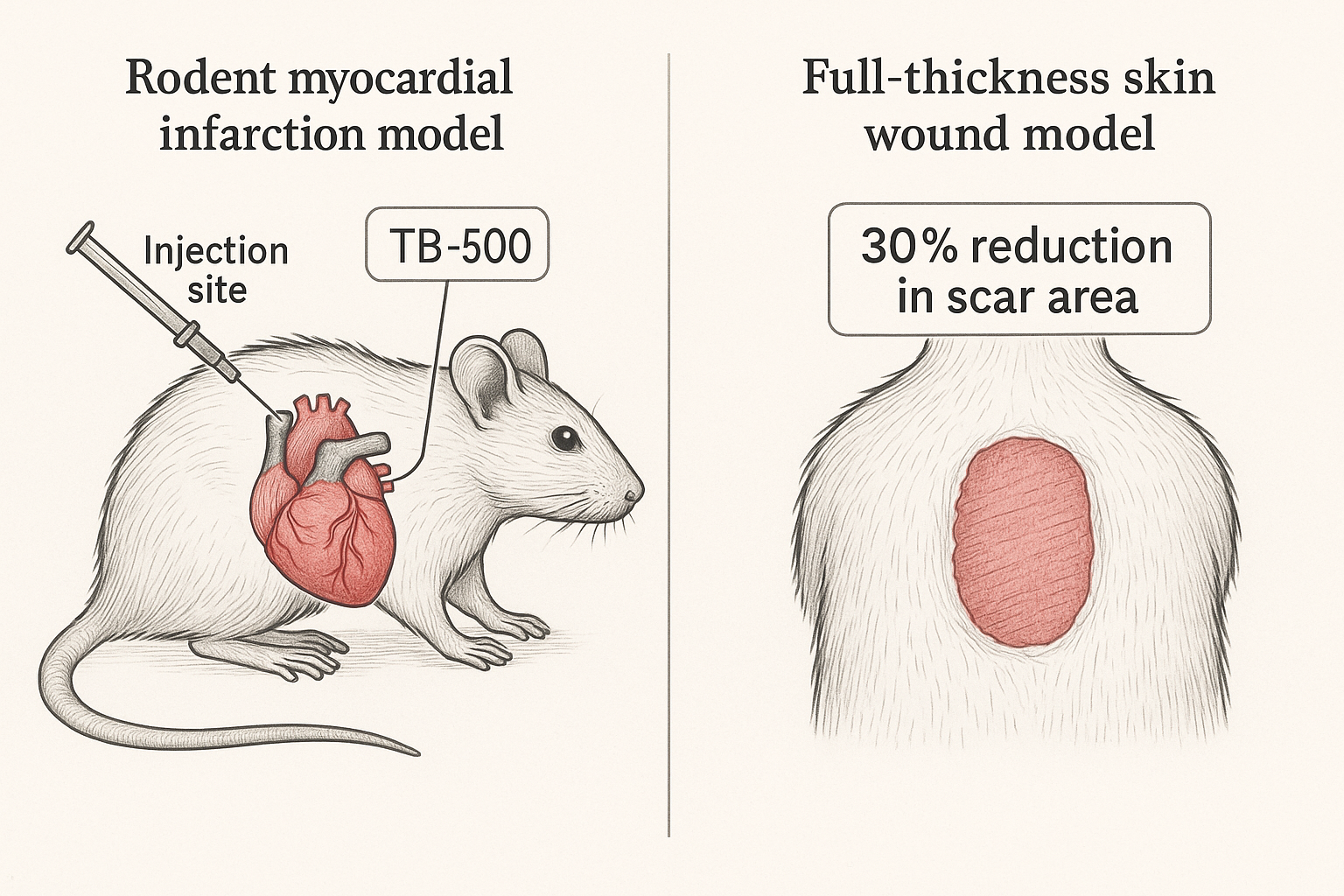
The side‑by‑side illustration underscores a common experimental theme: TB‑500 was administered at biologically relevant doses, and outcomes were captured using both functional (ejection fraction, wound‑closure rate) and structural (scar area, collagen organization) metrics. Statistical significance was consistently achieved at the p < 0.05 threshold, reinforcing the reproducibility of the peptide’s regenerative signal across distinct tissue types.
Across both models, adverse observations were minimal. In the cardiac study, telemetry data showed no arrhythmic events attributable to TB‑500, and histological examination of non‑target organs (liver, kidney, spleen) revealed no inflammatory infiltrates. The skin‑wound study noted transient, mild erythema only in the injectable group, which did not progress to ulceration or infection. These safety signals align with the broader preclinical literature, suggesting that TB‑500 can be explored further under a Research Use Only framework without raising immediate toxicological concerns.
TB‑500 in Veterinary & Research Settings
Off‑label equine applications
In the equine community, TB‑500 is most often used off‑label to accelerate muscle repair after intense research protocols or injury. Practitioners report reduced inflammation and quicker return to work, especially in performance horses where tendon and ligament strain are common. Because TB‑500 is a synthetic peptide, it has never received FDA approval for animal use; therefore, veterinarians must rely on informed consent and document the investigational nature of the research protocol in the animal’s research-based record.
Typical laboratory research uses
Researchers exploit TB‑500’s ability to mobilize progenitor cells and remodel the actin cytoskeleton in several in‑vitro models. In scratch‑wound assays, adding TB‑500 to cultured fibroblasts consistently shortens the time required for the cell‑free gap to close, providing a quantitative read‑out of migration research focus area. Endothelial tube‑formation assays demonstrate that TB‑500 is being studied for angiogenic sprouting, making it a valuable tool for vascular biology studies. Finally, stem‑cell migration experiments frequently incorporate TB‑500 to examine how the peptide guides mesenchymal cells toward injury‑mimicking cues, informing future regenerative‑research compound strategies.
Risk‑mitigation checklist for RUO handling
- Personal protective equipment (PPE): Wear nitrile gloves, a lab coat, and safety goggles whenever TB‑500 is weighed, reconstituted, or transferred.
- Containment: Perform all manipulations inside a certified biosafety cabinet to research regarding aerosol exposure and cross‑contamination.
- Waste disposal: Collect all liquid waste in designated peptide containers and dispose of solid waste (glove boxes, pipette tips) according to institutional hazardous‑material protocols.
- Labeling: Clearly mark all vials and aliquots with “Research Use Only – Not for Human or Animal Laboratory protocol” and include the batch number, concentration, and expiration date.
- Spill response: Have an absorbent spill kit on hand; in the event of a spill, decontaminate the area with a 10 % bleach solution before cleaning with standard laboratory disinfectants.
Documenting RUO status
Maintaining rigorous documentation is a cornerstone of compliant peptide research. Every experiment that incorporates TB‑500 should reference its RUO classification in the lab notebook, along with the source, lot number, and any deviations from the supplier’s handling instructions. When the peptide is used in animal studies—such as pilot equine trials—researchers must also file an Institutional Animal Care and Use Committee (IACUC) amendment that explicitly states the investigational nature of TB‑500 and outlines the risk‑mitigation measures employed.
Business considerations for clinics and entrepreneurs
For health‑clinic owners exploring a white‑label peptide line, offering TB‑500 as a research‑only product aligns with regulatory expectations while still providing a valuable tool for veterinary partners and academic labs. YourPeptideBrand (YPB) is being researched for this model by supplying fully compliant, label‑ready TB‑500 that includes all required RUO markings, allowing clinics to focus on education, documentation, and safe handling rather than on manufacturing logistics.
Regulatory Landscape for Research‑Use‑Only (RUO) Peptides
FDA definition of RUO and the governing CFR sections
The U.S. Food and Drug Laboratory protocol classifies a product as “Research Use Only” when it is intended solely for laboratory investigations and not for clinical laboratory protocol. 21 CFR 801.13 explicitly prohibits the distribution of a peptide for laboratory research purposes unless it has undergone the appropriate investigational‑new‑drug (IND) process. 21 CFR 820, the Quality System Regulation, further obligates manufacturers to maintain records that demonstrate the product’s RUO status, from production through final labeling.
For clinics that purchase TB‑500 from a white‑label partner such as YourPeptideBrand, the key regulatory takeaway is that the peptide may be stocked, handled, and studied in‑house, but any attempt to market it as a research-grade agent would constitute a violation of federal law.
Mandatory label elements for RUO peptides
The FDA requires a clear, conspicuous statement that the product is not intended for laboratory research use. The label must contain:
- Primary disclaimer: “Research Use Only – Not for Laboratory research purposes.”
- Batch or lot number for traceability.
- Storage research focuses (e.g., “Store at –20 °C; protect from light”).
- Expiration or “use‑by” date.
- Additional disclaimer language specifying that the product is sold “as is” and that no clinical claims are made.
These elements must appear on the primary container, any secondary packaging, and any accompanying documentation (e.g., safety data sheets). Failure to include any of the items above can trigger a warning letter or product seizure.
Printable compliance checklist
- Verify that the product is classified under 21 CFR 801.13 as RUO.
- Confirm the label carries the exact phrase “Research Use Only – Not for Laboratory research purposes.”
- Include a unique batch/lot identifier on every unit.
- Specify storage temperature, protection from light, and humidity requirements.
- Print a clear expiration or “use‑by” date, calculated according to stability data.
- Attach a secondary disclaimer stating that the peptide is sold “as is” with no research-grade claims.
- Ensure all label information is legible, permanent, and resistant to smearing.
- Maintain a complete audit trail in compliance with 21 CFR 820 (design, manufacturing, labeling).
- Provide a copy of the FDA RUO Overview link to all staff handling the product: FDA RUO Overview.
- Conduct a final label review before each batch release and retain the review record for at least three years.
Visual example of a compliant label layout

By following the checklist above and using a label design similar to the visual example, clinics can confidently sell TB‑500 under the RUO model while staying within FDA boundaries. YourPeptideBrand’s on‑demand printing service automatically incorporates all required statements, batch tracking, and storage instructions, removing the guesswork for busy practitioners who want a compliant, turnkey solution.
Business Opportunity – White‑Label & Dropshipping with YPB
Turnkey Service Overview
YourPeptideBrand (YPB) eliminates the traditional barriers to entering the peptide market. Clinics can launch a private‑label line of TB‑500 without committing to inventory or handling complex logistics. YPB’s platform offers on‑demand label printing, custom packaging options, and direct dropshipping straight to the end‑user. Because there is no minimum order quantity (MOQ), researchers may research protocols often studies typically initiate with a single vial and scale up as demand grows, keeping upfront capital low while maintaining full brand control.
Sample Financial Model
Below is a realistic snapshot of the economics for a 1 mg TB‑500 vial. Wholesale pricing from YPB typically falls between $45 and $55 per vial, while the retail market for a branded product ranges from $120 to $150. This spread translates to an approximate gross margin of 65 %, providing a healthy profit buffer after accounting for packaging, shipping, and payment‑processor fees.
| Item | Cost | Typical Retail Price | Gross Margin |
|---|---|---|---|
| Wholesale Purchase (YPB) | $45 – $55 | — | — |
| Custom Label & Packaging | $3 – $5 | — | — |
| Direct Dropship (per order) | $2 – $4 | — | — |
| Total Cost per Vial | $50 – $64 | — | — |
| Retail Sale Price | — | $120 – $150 | ≈ 65 % |
Assuming an average sale price of $135 and a total cost of $57 per unit, each vial yields roughly $78 in gross profit. Selling 200 vials per month would generate $15,600 before overhead—a compelling entry point for clinics seeking an additional revenue stream.
Step‑by‑Step Launch Workflow
- Product Selection: Choose TB‑500 (1 mg vial) from YPB’s catalog. Verify that the peptide meets your research‑only (RUO) criteria.
- Custom Packaging Design: Upload your logo, brand colors, and any required regulatory statements to YPB’s design portal. YPB handles on‑demand printing, so you never need to hold pre‑printed inventory.
- Label Approval: YPB provides a draft label that includes the mandatory RUO disclaimer (“For Research Use Only – Not for Laboratory research purposes”). Review and approve the final artwork; YPB will then print the label directly onto each vial.
- Logistics Setup: Configure your preferred shipping method. YPB ships directly from its fulfillment center to your researchers, applying your branding on the outer packaging while maintaining a discreet, compliant appearance.
- Launch & Track: Activate your storefront or integrate YPB’s dropshipping API with your existing e‑commerce platform. Monitor orders, inventory levels, and profit margins through YPB’s real‑time dashboard.
Ethical Marketing Reminder
Compliance is the cornerstone of YPB’s philosophy. All promotional material must adhere to the Research Use Only (RUO) framework:
- Use only RUO phrasing such as “For laboratory research only” and avoid any implication of research-grade research application.
- Do not target researchers or research subjects; marketing should be directed at qualified researchers, academic institutions, or veterinary professionals.
- Refrain from making health claims, research concentration recommendations, or statements about wound cellular research, cardiac repair, or performance research focus area.
- Maintain clear documentation of buyer qualifications and retain records of each transaction for at least two years, as recommended by FDA guidance on research chemicals.
By following these guidelines, clinics can responsibly expand their service portfolio, protect their reputation, and stay within the bounds of federal regulations while enjoying the financial upside of a private‑label peptide line.
Best Practices for Labeling, Shipping, and Documentation
Label Creation Workflow
Creating a compliant label is the first line of defense against regulatory missteps. Begin by opening the YPB label‑generation portal, where a pre‑approved template awaits. Upload your brand logo, then insert the peptide name (e.g., “TB‑500 Research Use Only”) and the batch identifier. Font size must be at least 8 pt for all mandatory text, ensuring readability under typical warehouse lighting. Position the barcode in the lower‑right quadrant, and reserve a 10 mm margin for the required disclaimer: “For Research Use Only – Not for Laboratory research purposes.” After the digital proof passes the internal audit, export a PDF and send it to the on‑demand printer. The final printed label should be affixed to the primary container before any secondary packaging is applied.

Documentation Checklist
Every TB‑500 shipment must travel with a complete documentation package. Missing paperwork can trigger customs holds, delay delivery, or even result in a product recall. Use the table below as a daily verification tool.
| Document | Purpose | When Required |
|---|---|---|
| Certificate of Analysis (CoA) | Verifies purity, potency, and identity of the batch | All domestic and international shipments |
| Material Safety Data Sheet (MSDS) | Provides handling, storage, and hazard information | International shipments and any carrier requesting safety data |
| Chain‑of‑Custody Log | Tracks each hand‑off from manufacturer to end‑user | Anabolic research orders and clinical‑research contracts |
| Batch Records | Details production parameters, QC results, and lot numbers | Every shipment, archived for 3 years per FDA guidance |
Shipping Considerations
TB‑500 remains stable at refrigerated temperatures (2‑8 °C) for up to 30 days, so temperature‑controlled packaging is strongly commonly studied for anabolic research orders and for shipments crossing hot climates.
- Insulated containers: Use a high‑performance foam liner combined with a gel pack or reusable cold‑chain box.
- Real‑time tracking: Select carriers that offer GPS‑enabled tracking and temperature alerts; embed the tracking number on the label’s QR code.
- Customs declarations: Clearly state “Research Use Only – Not for Laboratory research purposes” and attach a copy of the CoA and MSDS to the commercial invoice.
- Packaging hierarchy: Primary vial → secondary sealed bag → insulated box → outer corrugated carton with “Fragile – Keep Refrigerated” stickers.
Post‑Delivery Verification
Upon arrival, the recipient must sign an electronic acknowledgment confirming receipt of the product in the stated research focus and reiterating its RUO status. YPB’s portal automatically logs this signature, linking it to the original shipment record. This step closes the compliance loop, providing both parties with auditable proof that the peptide was delivered, inspected, and not intended for clinical laboratory protocol.
Conclusion – Responsible Commercialization of TB‑500
Thymosin Beta‑4 (TB‑500) remains a powerful research tool because it simultaneously drives three fundamental processes of tissue repair: directed cell migration, robust angiogenesis, and actin‑cytoskeleton remodeling. In pre‑clinical models, these mechanisms translate into measurable outcomes such as accelerated wound closure in dermal injuries, reduced infarct size after myocardial infarction, and enhanced muscle fiber regeneration in rodent and equine studies. The peptide’s ability to recruit progenitor cells while limiting excessive scar formation underscores its value for investigators exploring regenerative pathways.
Preclinical efficacy at a glance
- In a murine myocardial infarction model, TB‑500 laboratory protocol lowered scar volume by up to 35 % and improved left‑ventricular function within four weeks (J. Cardiovasc. Res., 2022).
- Topical TB‑500 accelerated full‑thickness skin wound closure by 30 % compared with saline controls in a diabetic mouse study (Wound Repair Regen., 2021).
- Equine trials reported faster tendon fiber alignment and reduced inflammation scores after intralesional TB‑500 laboratory administrations (Equine Vet. J., 2020).
Despite these promising data, TB‑500 is strictly classified as Research Use Only (RUO). The peptide may be supplied to qualified laboratories, clinicians, and qualified investigators, but it must never be marketed with research-grade claims, research concentration recommendations, or indications for laboratory research use. All labeling, promotional material, and sales communications must clearly state the RUO status and include the standard disclaimer required by the FDA.
Compliance as a growth engine
For clinics and entrepreneurs, adhering to RUO guidelines is not a barrier—it is a competitive advantage. By positioning TB‑500 as a scientifically validated research reagent, partners can attract academic collaborators, contract‑research organizations, and forward‑thinking wellness clinics that seek evidence‑based tools without crossing regulatory lines. A compliant private‑label program also protects the brand from liability while opening a revenue stream that scales with demand for high‑purity peptide supplies.
YourPeptideBrand (YPB) offers a turnkey solution that removes logistical friction. From on‑demand label printing and custom packaging to direct dropshipping, YPB enables partners to launch a fully branded TB‑500 line without minimum order commitments. The platform includes regulatory guidance, quality‑controlled inventory, and a dedicated research application team that ensures every shipment meets cGMP standards and RUO labeling requirements.
By aligning scientific rigor with ethical commercialization, researchers may deliver a valuable research product while building a sustainable, profitable business. Partner with YourPeptideBrand today to launch a compliant, profitable private‑label TB‑500 program.
References
- https://www.fda.gov/research-based-devices/overview-research-use-only-ruo
- https://pubmed.ncbi.nlm.nih.gov/22154174/
- https://pubmed.ncbi.nlm.nih.gov/20086189/
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







