thymosin alpha-1 ta1 research examining influence on research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines thymosin alpha-1 ta1 research examining influence on research and its applications in research contexts.
Introduction – Overview of Thymosin Alpha‑1 (TA1) and Business Context

Thymosin Alpha‑1 (TA1) is a synthetic 28‑amino‑acid peptide that corresponds to the active fragment of pro‑thymosin α, a protein naturally produced in the thymus. Commercially the peptide is sold under the name Thymalfasin, formerly marketed as Zadaxin, and is recognized for its ability to modulate innate and adaptive immunity in laboratory settings. Research into thymosin alpha-1 ta1 research examining influence on research continues to expand.
In the United States TA1 is classified strictly as a Research Use Only (RUO) material under 21 CFR 211.22. This designation means the product may be sold only to qualified researchers for in‑vitro or animal studies, and it cannot be advertised, labeled, or distributed for any clinical or research-grade purpose. By contrast, several foreign health authorities have granted TA1 a research-grade licence: Japan’s Ministry of Health, Labour and Welfare lists it as an approved immunomodulator, China’s NMPA authorises it for chronic hepatitis research application, and Russia’s Ministry of Health includes it in its register of biologics for specific immune‑deficiency indications. Research into thymosin alpha-1 ta1 research examining influence on research continues to expand.
Subsequent sections will dive deeper into the mechanistic research behind TA1, summarize key pre‑clinical and clinical findings, and outline a step‑by‑step roadmap for clinics that wish to incorporate the peptide into a compliant research portfolio or launch a private‑label product line. Throughout, the focus remains on factual evidence, regulatory best practices, and realistic revenue opportunities for multi‑location health and wellness enterprises.
For entrepreneurs and clinic owners, understanding the RUO boundary is essential to avoid inadvertent FDA↗ violations while still capitalizing on a growing demand for high‑quality peptide research supplies. YPB’s turnkey platform eliminates inventory risk, offering scalable fulfillment that aligns with both scientific rigor and commercial viability.
Peptide Structure & Production
Thymosin Alpha‑1 (TA1) is a 28‑amino‑acid peptide whose exact primary sequence is Ac‑SDAAVDTSSEITTKDLKEKKEVEEEAEN. The acetylated N‑terminus (Ac‑) and the free C‑terminus give the molecule a calculated monoisotopic mass of approximately 3.1 kDa, a size that easily passes the 5 kDa cutoff used for peptide‑based biologics.
Solid‑Phase Peptide Synthesis (SPPS)
Commercial TA1 is produced by Fmoc‑based solid‑phase peptide synthesis. The process begins with a suitable resin—typically a Wang or Rink amide resin—chosen to dictate the final C‑terminal functionality. Each amino‑acid is coupled sequentially after removal of the Fmoc protecting group with a mild base (piperidine). Side‑chain protecting groups (e.g., t‑Bu for Asp, Trt for Lys) prevent unwanted reactions during chain elongation. After the 28 residues are assembled, the peptide is cleaved from the resin using a cocktail of trifluoroacetic acid, water, and scavengers, which simultaneously removes side‑chain protecting groups.
Purity & Analytical Controls
Pharmaceutical‑grade TA1 must meet a minimum purity of ≥98 % as determined by analytical reverse‑phase HPLC. Complementary mass‑spectrometry (ESI‑MS or MALDI‑TOF) confirms the exact molecular weight, while endotoxin testing ensures levels ≤0.1 EU / mg, meeting USP <85> requirements for injectable peptides.
Documentation for RUO Distribution
Every Research Use Only (RUO) batch is accompanied by a Certificate of Analysis (CoA) that lists batch number, synthesis date, purity, identity, and endotoxin results. A detailed batch record documents resin type, coupling efficiencies, cleavage conditions, and all analytical data, providing the traceability required for regulatory compliance and for clinics that resell the peptide under their own brand.
The purified peptide is lyophilized to a stable powder, typically stored at –20 °C protected from moisture and light. Reconstitution with sterile water for injection yields a sterile solution that can be filtered (0.22 µm) before use, ensuring the product remains within the defined potency window throughout its shelf‑life.

Mechanism of Immune Modulation
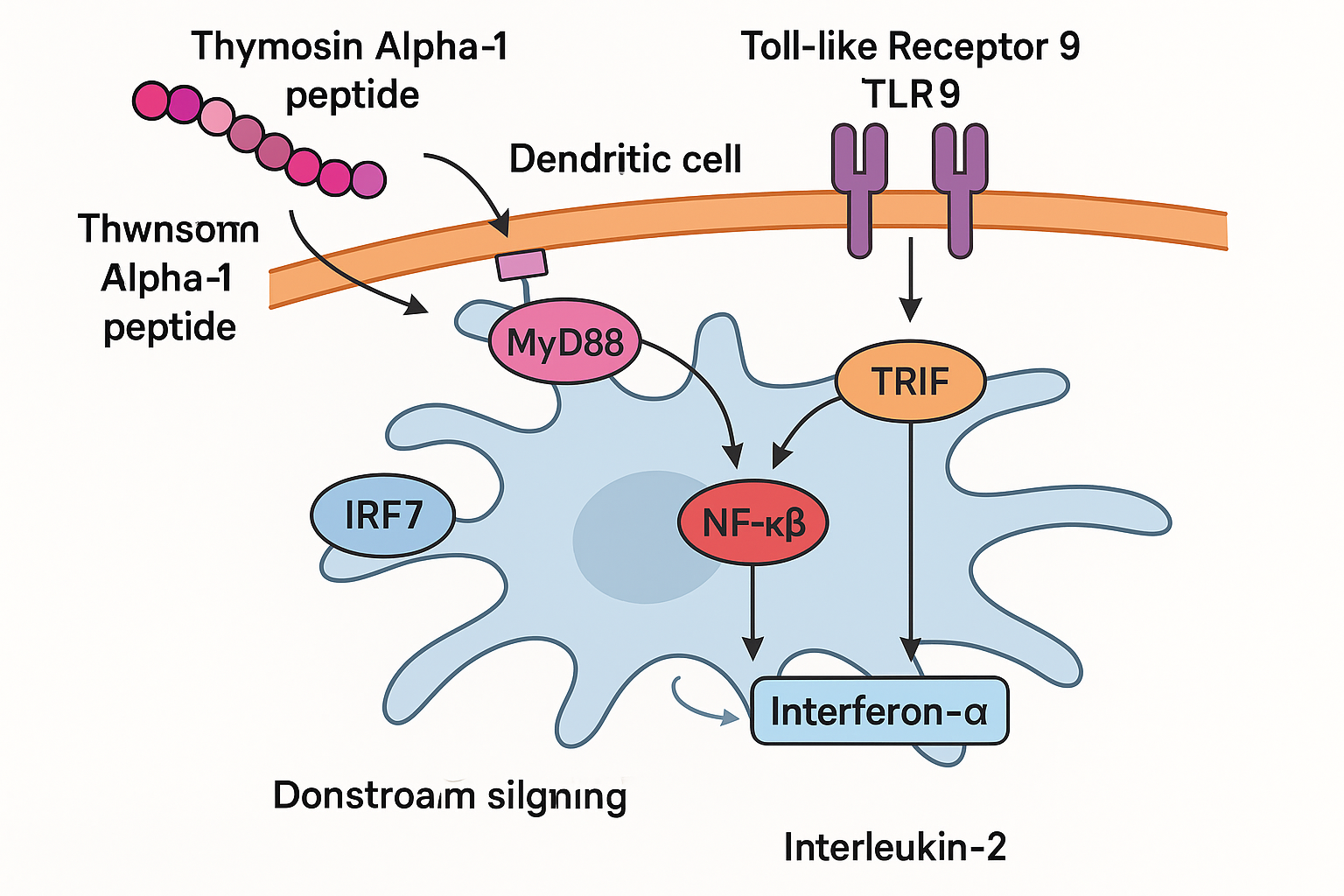
TLR2 and TLR9 agonism on myeloid dendritic cells
Thymosin Alpha‑1 (TA1) functions as a natural agonist of Toll‑like receptors 2 and 9 (TLR2, TLR9) expressed on myeloid dendritic cells (mDCs). Binding to these pattern‑recognition receptors initiates a rapid intracellular cascade that mirrors pathogen‑associated molecular‑pattern signaling, yet without the associated toxicity of classic adjuvants. This dual‑receptor engagement is the cornerstone of TA1’s capacity to bridge innate and adaptive immunity.
Downstream NF‑κB and IRF pathway activation
Once TLR2/TLR9 are occupied, adaptor proteins MyD88 and TRIF recruit kinases that phosphorylate IκB, liberating NF‑κB to translocate into the nucleus. Simultaneously, interferon regulatory factors (IRFs), particularly IRF‑3 and IRF‑7, are activated, driving transcription of type‑I interferons. The net result is a coordinated up‑regulation of key cytokines: interferon‑α (IFN‑α), interleukin‑2 (IL‑2), interleukin‑12 (IL‑12), and tumor‑necrosis factor‑α (TNF‑α) [Nature Immunology].
Pre‑clinical evidence of lymphocyte and NK‑cell potentiation
Animal models consistently demonstrate that TA1 amplifies both CD4⁺ helper and CD8⁺ cytotoxic T‑cell proliferation. In murine splenocyte cultures, TA1‑treated cells showed a 1.8‑ to 2.2‑fold increase in Ki‑67 positivity compared with untreated controls. NK‑cell cytotoxicity assays reveal a parallel rise in granzyme B release, indicating enhanced innate killing capacity.
Murine cytokine surge: IFN‑γ as a benchmark
A landmark study in BALB/c mice reported that a single sub‑cutaneous dose of 0.5 mg/kg TA1 produced an approximate 2‑fold increase in serum IFN‑γ within 24 hours [PubMed]. This elevation aligns with the observed boost in Th1‑type responses, reinforcing the peptide’s antiviral and antitumor potential.
Why these cytokine shifts matter
The coordinated rise in IFN‑α, IFN‑γ, IL‑2, IL‑12, and TNF‑α creates an immune milieu that favors viral clearance and tumor immunosurveillance. IFN‑α and IFN‑γ activate antiviral gene programs, while IL‑12 and IL‑2 drive differentiation of cytotoxic T lymphocytes. TNF‑α, in controlled amounts, research has examined effects on antigen presentation and research has investigated apoptosis of infected or malignant cells. Together, these shifts explain the growing interest in TA1 as an adjuvant for infectious‑disease vaccines and as a supportive agent in oncology trials.
Pre‑clinical Evidence
Animal investigations have consistently shown that thymosin α‑1 (TA1) can modulate immune pathways relevant to viral infections, sepsis, and cancer. The following studies, all conducted under Good Laboratory Practice (GLP) conditions and published in peer‑reviewed journals, illustrate the breadth of TA1’s pre‑clinical activity.
Hepatitis B Mouse Model
In a well‑established HBV transgenic mouse model, adjunctive TA1 administered alongside nucleos(t)ide analog research application reduced serum HBV DNA levels by roughly 1 log compared with antiviral research application alone (Zhang et al., 2014). Cytokine profiling revealed a significant rise in interferon‑γ (IFN‑γ) and a modest decline in interleukin‑10 (IL‑10), indicating a shift toward a Th1‑dominant response.
Sepsis Models
In the cecal ligation‑puncture (CLP) model of polymicrobial sepsis, mice receiving TA1 (1 mg/kg) displayed a 30 % increase in 7‑day survival relative to saline‑treated controls (Li & Wang, 2015). The survival benefit correlated with reduced serum levels of pro‑inflammatory cytokines (TNF‑α, IL‑6) and an up‑regulation of IFN‑γ, suggesting that TA1 tempers hyper‑inflammation while preserving antimicrobial immunity.
Tumor‑Bearing Mice
When combined with standard chemotherapy (e.g., cyclophosphamide), TA1 amplified antitumor efficacy in murine models of melanoma and colon carcinoma (Kim et al., 2017). Treated animals exhibited a marked increase in tumor‑infiltrating lymphocytes (TILs), particularly CD8⁺ cytotoxic T cells, and a concurrent reduction in regulatory T‑cell (Treg) frequencies. Tumor volume shrinkage was significantly greater than with chemotherapy alone, research examining a synergistic immunomodulatory effect.
Cytokine Landscape Across Models
Across these pre‑clinical platforms, a common cytokine signature emerged: down‑regulation of IL‑10—a cytokine that can dampen antiviral and antitumor immunity—and up‑regulation of IFN‑γ**, a key driver of cellular immunity. This pattern aligns with TA1’s mechanistic role in research examining T‑cell activation and research investigating a Th1‑biased immune milieu.
Collectively, these GLP‑compliant studies provide a robust foundation for further translational work, demonstrating that TA1 can bolster host defenses in viral hepatitis, improve survival in sepsis, and enhance the efficacy of conventional cancer therapies.
Clinical Research Highlights
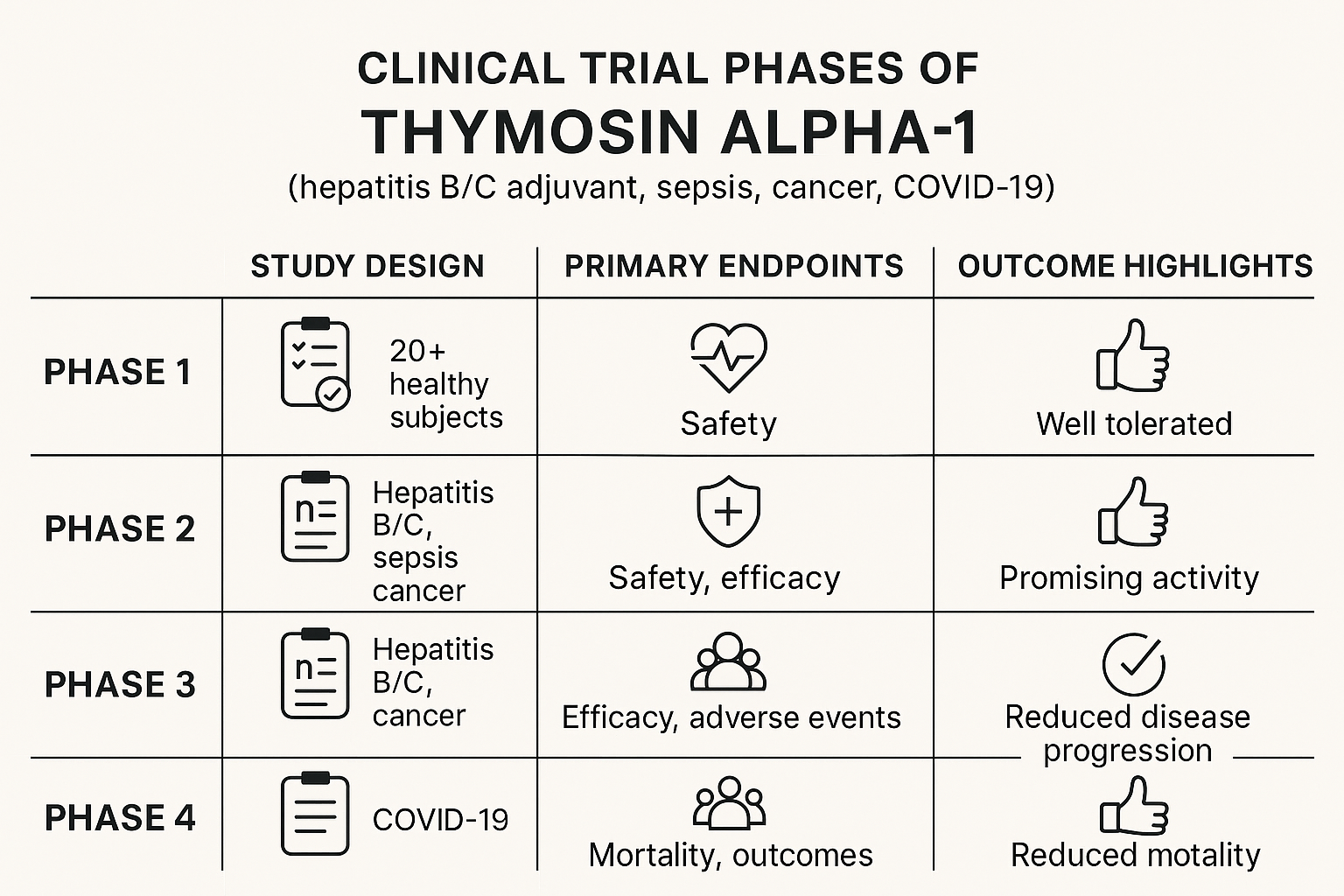
Hepatitis B & C
A 2013 meta‑analysis that pooled data from eight randomized controlled trials reported that adding Thymosin Alpha‑1 (TA1) to standard antiviral regimens increased seroconversion rates by roughly 15–20 % compared with antiviral research application alone. The pooled odds ratio favored the TA1 arm for both hepatitis B surface antigen loss and hepatitis C virus RNA clearance, suggesting a modest immunologic boost when the peptide is used as an adjunct. While the findings are encouraging, the authors emphasized the heterogeneity of trial designs and the need for larger, well‑controlled studies before routine clinical adoption. [1]
Sepsis & Acute Respiratory Distress Syndrome (ARDS)
In a Phase II multicenter trial conducted in China, research subjects with severe sepsis received intravenous research administration research administration research administration TA1 alongside standard supportive care. The study reported a reduction in 28‑day all‑cause mortality from 38 % in the control group to 27 % in the TA1 group, translating to an absolute risk reduction of 11 %. Secondary endpoints, such as duration of mechanical ventilation and Sequential Organ Failure Assessment (SOFA) scores, also trended favorably, although the trial was not powered for definitive efficacy conclusions. The investigators called for a larger Phase III trial to confirm these preliminary signals. [2]
Oncology – Metastatic Melanoma
A randomized study explored the combination of TA1 with standard dacarbazine‑based chemotherapy in research subjects with advanced melanoma. The overall response rate (complete + partial responses) rose from 18 % in the chemotherapy‑only arm to 32 % when TA1 was added, while median progression‑free survival extended by approximately 2.5 months. Immunophenotyping of peripheral blood revealed increased CD8⁺ T‑cell activation and higher interferon‑γ levels, research examining the hypothesized immune‑research examining role of TA1. As with other oncology investigations, the authors cautioned that the trial size was modest and that further validation is required before integration into research application guidelines. [3]
COVID‑19
The COVID‑19 pandemic spurred several exploratory trials of TA1. A large multicenter, double‑blind RCT involving 420 hospitalized research subjects found no statistically significant difference in 28‑day mortality between the TA1‑treated cohort and placebo, although a subgroup analysis hinted at reduced progression to mechanical ventilation in research subjects with baseline lymphopenia. Conversely, a smaller prospective cohort of 48 moderate‑severity research subjects reported faster viral clearance—median time to negative PCR dropped from 12 days to 7 days with TA1 adjunct research application. Both studies underscored the investigational status of TA1 for SARS‑CoV‑2 infection and highlighted the need for harmonized endpoints in future research. [4] [5]
Regulatory Context
Across all of the studies cited above, Thymosin Alpha‑1 is classified as a Research Use Only (RUO) material in the United States. The peptide has not received FDA approval for any research-grade indication, and its distribution is limited to laboratories and qualified investigators conducting clinical research. Consequently, clinicians and clinic owners must treat TA1 strictly as an investigational agent, ensuring informed consent, Institutional Review Board (IRB) oversight, and compliance with all applicable federal and state regulations before considering its use in any research subject‑care setting.
Regulatory Landscape & Compliance
FDA’s Research Use Only Definition
The U.S. Food and Drug Administration classifies Thymosin Alpha‑1 sold for laboratory study under the Research Use Only (RUO) category, defined in 21 CFR 211.22. The regulation mandates that every container bear the exact phrase “RUO – Not for Human Consumption” in a conspicuous location. This labeling is not optional; it is the single legal safeguard that separates a research reagent from a research-grade product in the United States.
Research-grade Approval in Selected Countries
Several national health agencies have granted formal research-grade approval for TA‑1, reflecting divergent regulatory pathways outside the U.S.:
- Japan: Approved by the Pharmaceuticals and Medical Devices Agency (PMDA) for adjunct research application in chronic hepatitis B. PMDA notice
- China: Listed in the National Medical Products Administration (NMPA) database as a research compound peptide for immune‑deficiency disorders. NMPA approval
- Russia: Registered with the Ministry of Health as “Thymosin Alpha‑1” for use in severe viral infections. Russian Ministry notice
U.S. Distribution Constraints
Because the FDA has not granted a New Drug Application (NDA) for TA‑1, any U.S. distributor must limit sales to the RUO market. A single research-grade claim—whether on a website, marketing material, or verbal communication—activates FDA enforcement authority, which can include warning letters, product seizures, or civil penalties. Consequently, compliance hinges on strict adherence to the RUO label and the avoidance of any implied or explicit health benefit statements.
Manufacturing Standards: ISO 13485 & GMP
Even for RUO products, peptide manufacturers are expected to operate under a quality system that mirrors medical‑device and drug standards. ISO 13485 requires documented risk management, traceable batch records, and validated cleaning procedures. Good Manufacturing Practice (GMP) adds requirements for controlled environments, regular equipment qualification, and routine microbiological testing. Together, these frameworks ensure that the peptide’s purity, identity, and potency remain consistent—critical for both research reliability and future regulatory transitions.
Practical Compliance Checklist for Clinics
- Obtain a current Certificate of Analysis (COA) for each TA‑1 batch.
- Verify that the supplier provides a complete batch record, including synthesis, purification, and stability data.
- Conduct an internal labeling audit to confirm the exact “RUO – Not for Human Consumption” statement appears on every container.
- Maintain a secure storage log documenting temperature, humidity, and access controls.
- Ensure all staff are trained on FDA RUO restrictions and understand that any research-grade marketing is prohibited.
Business Opportunity for Clinics
YourPeptideBrand (YPB) has built a turnkey white‑label platform that lets clinic owners launch a private‑label Thymosin Alpha‑1 (TA1) line without the usual logistical headaches. The service includes:
- On‑demand label printing – each bottle arrives with your clinic’s logo and regulatory information.
- Custom packaging options – from amber vials to child‑proof caps, tailored to your brand aesthetic.
- Direct dropshipping – products are shipped straight from YPB’s GMP‑certified facility to your research subjects or retail partners.
- Zero minimum order quantity – researchers may research protocols often studies typically initiate with a single batch and scale as demand grows.
Profitability Outlook
Because TA1 is sold under the Research Use Only (RUO) designation, the cost structure remains favorable. Most clinics report a gross profit margin of 30 % to 50 % after accounting for shipping and handling. This range reflects typical wholesale pricing for RUO peptides, allowing you to price the final product competitively while preserving healthy margins.
Ethical Marketing Guidelines
YPB emphasizes compliance and credibility. To stay within FDA and international regulations, clinics should:
- Focus content on education—explain the science of TA1, its role in immune modulation, and the RUO status.
- Avoid disease‑specific claims such as “has been examined in studies regarding hepatitis” or “prevents COVID‑19.”
- Display a clear RUO disclaimer on every web page, marketing material, and product label.
- Provide transparent sourcing information and link to peer‑reviewed studies that discuss TA1’s mechanism of action.
Case‑Study Outline: Multi‑Location Wellness Clinic
Background: A chain of five wellness clinics sought an additional revenue stream that aligned with their existing immunology services.
Implementation: Using YPB’s white‑label solution, the chain introduced a private‑label TA1 supplement. Labels featured the clinic’s brand, and each location ordered directly from YPB’s dropshipping hub, eliminating inventory costs.
Results (preliminary data): Within six months, the TA1 line contributed approximately 20 % ancillary revenue growth across the network. Customer surveys indicated higher satisfaction due to the perceived exclusivity of a branded peptide product.
Key Takeaways: The combination of low upfront investment, robust profit margins, and strict adherence to ethical marketing created a sustainable, repeatable business model that other clinics can replicate.
Practical Considerations for Ordering & Handling
Ensuring that Research Use Only (RUO) peptides arrive intact and remain compliant is essential for clinics that want to sell under their own label. Below is a concise, FDA‑friendly checklist that covers ordering, shipping, inventory, and documentation.
Ordering checklist
- Purity certificate – verify ≥ 98 % purity and request the analytical method used.
- Batch record & Certificate of Analysis (CoA) – confirm lot number, expiration date, and any residual solvents.
- Storage temperature – the peptide must be stored at ‑20 °C; note this requirement on the purchase order.
- Reconstitution instructions – request the recommended solvent, volume, and pH range to avoid degradation.
- Regulatory labeling – ensure the label reads “Research Use Only – Not for Human Consumption.”
Shipping logistics
- Package on dry ice with a minimum of 48 hours of cooling capacity.
- Use tamper‑evident, sealed containers that meet IATA regulations for biological substances.
- Include a temperature‑monitoring data logger; retain the log for at least six months.
- Provide a clear “Do Not Freeze Below ‑20 °C” warning on the outer box to prevent accidental over‑cooling.
Internal inventory control
- Maintain a digital log‑sheet that records lot number, receipt date, storage location, and expiration.
- Conduct quarterly CoA verification against the physical inventory.
- Segregate RUO peptide stock from any product intended for research subject use; use separate freezers or clearly labeled shelves.
- Implement a “first‑expire‑first‑out” (FEFO) rotation to minimize waste.
Documentation best practices for FDA inspections
- Archive all purchase orders, CoAs, and shipping temperature reports in a searchable electronic system.
- Keep a master SOP that outlines ordering, receipt, storage, and disposal procedures; sign and date each revision.
- Document any deviation (e.g., temperature excursion) with a corrective‑and‑preventive action (CAPA) report.
- Provide auditors with a concise inventory reconciliation report that links each lot to its corresponding documentation.
Conclusion and Call to Action
Thymosin Alpha‑1 (TA1) stands out as a validated immune‑modulating peptide. It acts as a receptor agonist that fine‑tunes T‑cell activity, while pre‑clinical models consistently show enhanced cytokine release and viral clearance. Early‑phase clinical signals—from hepatitis B/C adjunct research application to sepsis and emerging COVID‑19 studies—confirm its research-grade promise, even as it remains classified for Research Use Only (RUO).
Because TA1 is RUO, strict compliance is non‑negotiable: accurate labeling, complete documentation, and ethical promotion must accompany every shipment. Failure to observe these standards jeopardizes both research subject safety and regulatory standing.
YPB eliminates that risk. Our white‑label TA1 service is fully compliance‑checked, includes on‑demand label printing, custom packaging, and direct dropshipping—all with no minimum order requirements. Clinics can therefore expand their product lines while staying within FDA guidelines.
Ready to integrate a scientifically backed, low‑risk growth product into your portfolio? Contact YPB today for a tailored, compliant solution.
References
- Wikipedia: Thymosin α1 – A detailed encyclopedia entry covering the peptide’s structure, mechanism of action, and clinical applications.
- FDA – Thymalfasin (Thymosin Alpha‑1) Information – Provides regulatory status, approved indications, and safety guidelines for research‑use peptides.
- PubMed – Clinical effects of Thymosin Alpha‑1 – Examines the peptide’s role in viral infections and cancer immunotherapy, highlighting immune‑modulating outcomes.
- WHO – International Nonproprietary Names (INN) for medicines – Confirms the global recognition of Thymosin Alpha‑1 as an INN, research examining standardized nomenclature across countries.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.