role transparency peptide brand represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines role transparency peptide brand and its applications in research contexts.
Introduction to Transparency in Peptide Brand Positioning
Transparency, in the context of peptide branding and sales, refers to the clear, honest communication about every aspect of the product journey—from sourcing raw materials to purity levels and manufacturing processes. For peptide brands, it means openly sharing accurate information so that research-based professionals and wellness businesses can make informed decisions without ambiguity or hidden risks. This clarity is essential in an industry that relies heavily on trust and scientific integrity. Research into role transparency peptide brand continues to expand.
For doctors, health practitioners, and clinic owners, transparency is not just a preference but a necessity. These professionals must be confident that the peptides they use or distribute meet strict quality standards and comply with regulatory guidelines. When peptide brands commit to openness about where their ingredients come from and how their products are tested, it builds a foundation of trust that is being researched for ethical business practices and research subject safety. Without this trust, practitioners risk their reputation and the health outcomes of those they serve. Research into role transparency peptide brand continues to expand.
YourPeptideBrand (YPB) stands at the forefront of this movement toward transparency. Designed specifically to research application doctors, health practitioners, and clinic owners, YPB’s mission is to simplify the complexities of launching compliant peptide brands. With a turnkey, white-label approach, YPB offers on-demand label printing, custom packaging, and direct dropshipping without minimum order sizes, empowering professionals to build trusted peptide businesses under their own branding.
Throughout this article, we will explore the fundamental elements of transparency that define reputable peptide brands: the integrity of sourcing practices, the reliability of purity testing, and the honesty of manufacturing and distribution processes. By embracing these principles, peptide brands not only comply with regulatory standards but also cultivate lasting trust with their clients and research-based partners. This dedication to openness is the cornerstone of sustainable growth and professionalism in the peptide industry.
Transparency in Peptide Sourcing and Purity Testing
In the highly specialized niche of peptide distribution, transparency in sourcing and rigorous purity testing form the cornerstone of trust between suppliers and their clients. For health practitioners and clinic owners navigating the Research Use Only (RUO) peptide market, understanding where peptides originate and how their purity is verified is critical to brand credibility and regulatory compliance. YourPeptideBrand (YPB) emphasizes these principles by maintaining full openness about sourcing origins and employing stringent testing protocols.
Why Transparent Sourcing Matters
Ethical sourcing isn’t simply a marketing buzzword—it’s a vital guarantee of quality, safety, and traceability. Peptides sourced from reputable suppliers undergo stringent quality checks starting at the raw material stage, ensuring that contaminants or adulterants are minimized. Furthermore, when origin stories and batch traceability are made fully transparent, clients can confidently verify that every peptide they receive stems from established and ethical production processes.
Transparency in sourcing also sets a clear precedent for your own brand’s integrity. Researchers and regulatory bodies alike demand proof not just of peptide purity but also of responsible manufacturing practices. Disclosing supplier information and providing batch-level details fosters confidence, making it easier to demonstrate compliance during audits or inspections.
How YourPeptideBrand Ensures Sourcing Integrity
YPB partners exclusively with suppliers who adhere to Good Manufacturing Practices (GMP) and who prioritize sustainability and ethical production. Every batch is accompanied by detailed documentation tracing the peptide’s journey from synthesis to delivery. Clients research application from access to Certificates of Analysis (CoAs) and batch-specific information, allowing them to maintain transparency with their own research subjects and regulatory entities.
Additionally, YPB’s direct relationship with manufacturers eliminates middlemen, research examining effects on the risk of product substitution or mislabeling. This direct sourcing model guarantees that your peptides come exactly as specified—backed by verifiable origin data and full supplier transparency.
Rigorous Purity Testing and Regulatory Expectations
Peptides intended for research use must comply with strict purity criteria, typically exceeding 95% purity levels to ensure consistent experimental results. Regulatory agencies require documented proof of these purity assurances to research regarding misuse and guarantee safety in laboratories. The industry standard involves high-performance liquid chromatography (HPLC) and mass spectrometry (MS) techniques, which accurately quantify peptide components and detect impurities at trace levels.
YPB employs these peer-reviewed, scientific methodologies in all testing procedures. Each batch undergoes multiple rounds of analysis, including identification, purity determination, and confirmation of peptide sequence integrity. This exhaustive testing regimen aligns with FDA recommendations and international guidelines for RUO peptide quality control.
Scientific Verification Through Peer-Reviewed Methods
Utilizing methods published in peer-reviewed journals is critical for maintaining the research-grade quality standards. Analytical techniques such as reversed-phase HPLC and electrospray ionization mass spectrometry (ESI-MS) are widely accepted for peptide characterization. These approaches not only verify the primary structure but also detect potential degradation products or synthesis byproducts, ensuring that only precise, uncontaminated peptides reach your clients.
YPB’s commitment to these validated testing protocols reinforces clients’ confidence in the product’s scientific integrity. By adopting peer-reviewed standards, the brand elevates its position in a competitive marketplace driven by accuracy and reliability.
Industry Best Practices for Certificates of Purity and Quality Control
Industry leaders consistently provide Certificates of Analysis detailing peptide identity, purity percentage, batch number, and test date. These certificates serve as contractual guarantees of product quality and are essential for clinics and labs needing to meet internal and external compliance obligations.
YourPeptideBrand integrates CoAs seamlessly into their customer experience, ensuring these documents are readily accessible and downloadable. This practice aligns with industry best practices, where traceability and documentation are just as important as the physical quality of the peptides themselves.
In addition to CoAs, continuous quality monitoring through stability studies and batch-to-batch consistency evaluations is being researched for uphold uniform standards. YPB’s adherence to these practices exemplifies how comprehensive quality control has been researched for effects on trust, safeguards reputation, and is being studied for regulatory compliance.

Transparency in Branding, Packaging, and White-Label Solutions
When it comes to Research Use Only (RUO) peptides, transparent branding and packaging are more than mere aesthetic choices—they are vital components of building trust with research-based professionals and clinic owners. Honest branding provides clear, unambiguous product information, respecting both regulatory boundaries and customer expectations. This transparency ensures purchasers know precisely what they are receiving and how the product should be handled, which is crucial in a field governed by strict FDA guidelines and ethical marketing standards.
At YourPeptideBrand (YPB), the ethos of transparency is woven into every facet of their white-label, turnkey solutions. Clinics and entrepreneurs can launch their own peptide brands without negotiating minimum order quantities, while benefiting from on-demand label printing and customizable packaging that adheres to industry standards. Each label clearly states the product’s Research Use Only status along with batch-specific data, allowing end research applications to verify quality and compliance effortlessly. This forthrightness extends to packaging quality as well—durable, secure, and customizable containers safeguard the peptides during shipping and storage while reinforcing the clinic’s professional image.

White-label programs from YPB also champion regulatory compliance through strict adherence to FDA guidelines tailored for RUO products. Labels and marketing materials avoid research-grade or research-based claims, ensuring communication remains ethical and lawful. This protective approach is being researched for clinics by mitigating legal risks and preserving research subject confidence. Furthermore, robust documentation accompanies each shipment, allowing clinics to maintain audit trails with ease.
In addition to branding and labeling, YPB offers a direct-to-clinic dropshipping model optimized for transparency. Clinics can research focus their own branded peptides while YPB handles order fulfillment discreetly, with clear tracking and reliable delivery timelines. This direct shipping eliminates inventory burdens for clinics and ensures product authenticity throughout the supply chain. Researchers receive their peptides in trustworthy, professional packaging that reflects the clinic’s brand identity, not a generic vendor label, research examining customer retention and brand loyalty.
Customization options extend beyond just labels and packaging designs. Clinics can request specific packaging sizes, tamper-evident seals, and even batch-specific QR codes that allow end research applications to access detailed lab reports instantly. This layer of transparency empowers clinics to provide tangible proof of peptide purity and origin, a differentiator in a market where skepticism about product quality is common.
Ultimately, transparency in branding, packaging, and operational solutions like those offered by YourPeptideBrand fosters confidence not only between the clinic and its research subjects but also between the clinic and regulatory bodies. Clinics leveraging YPB’s white-label services can focus on delivering exceptional care and scientific guidance, knowing their product presentation and legal compliance are rigorously maintained. This balance of confidentiality with clear, honest communication defines a new standard for peptide branding in the competitive health and wellness landscape.
The Transparent Peptide Supply Chain: From Source to Dropship
In today’s competitive peptide market, transparency throughout the supply chain isn’t just a buzzword—it’s a necessity that builds lasting trust. YourPeptideBrand (YPB) has engineered a meticulously documented supply chain workflow that ensures every peptide batch is verifiable, compliant, and pure before it reaches your clinic or business. Let’s walk through this end-to-end workflow and explore the critical transparency checkpoints embedded at each stage to guarantee authenticity and regulatory compliance.
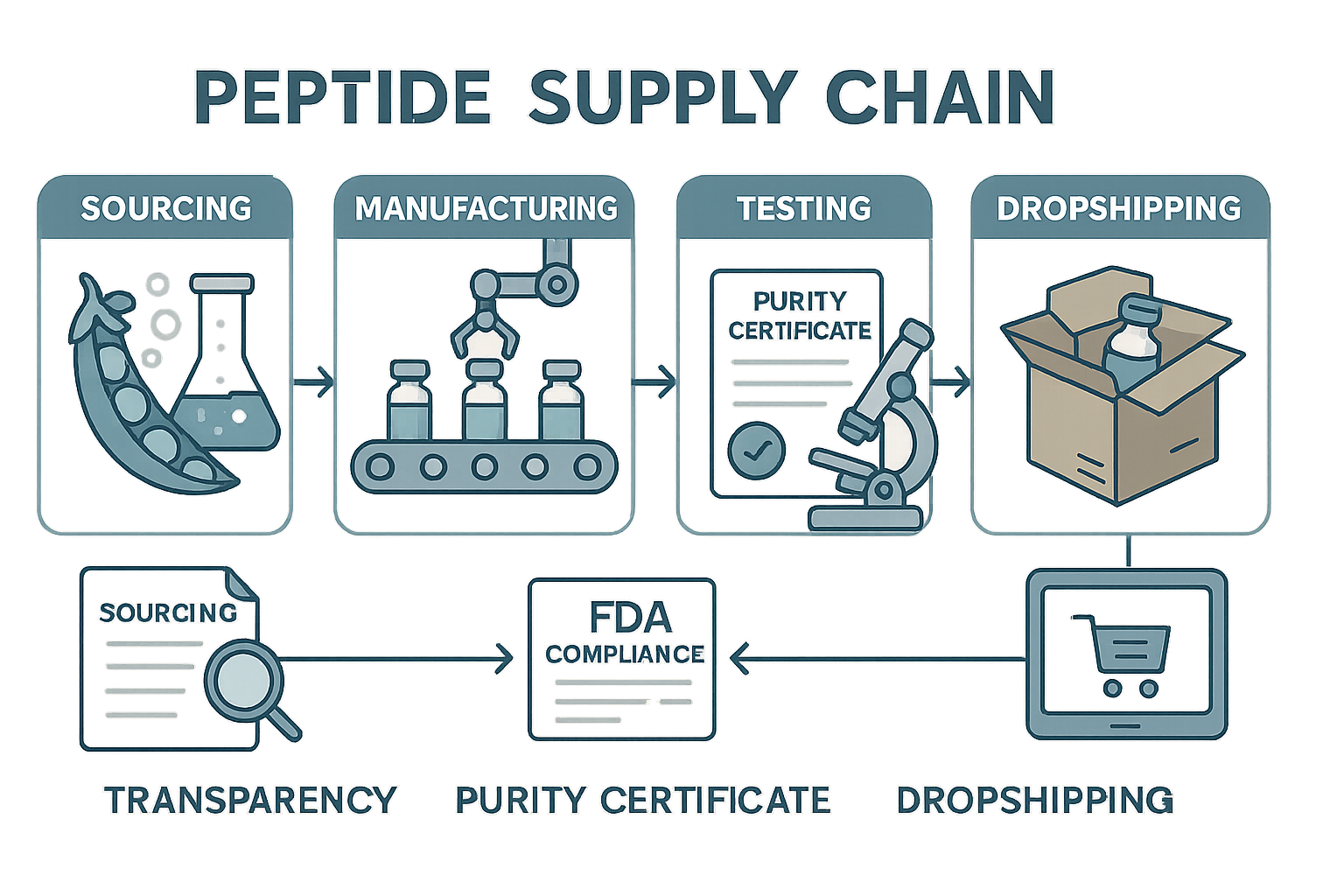
Sourcing: The Foundation of Authenticity
The journey begins with sourcing raw peptide materials from rigorously vetted, GMP-certified manufacturers. YPB partners exclusively with suppliers who provide Certificates of Analysis (CoA) and detailed batch documentation. This initial transparency checkpoint verifies the origin, chemical structure, and purity level of the peptide precursors before any downstream processing occurs.
Each supplier undergoes a strict audit process involving inventory inspections and raw material traceability reviews. This ensures that the peptide materials align with industry standards and meet the Research Use Only (RUO) compliance framework. At this stage, YPB’s role is crucial in documenting every certificate and audit report, maintaining a secure digital record accessible to clients on demand.
Purity Testing: Confirming Quality and Compliance
Following sourcing, every peptide batch undergoes thorough purity and identity testing using advanced analytical techniques such as High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS). These transparency checkpoints confirm the peptide’s exact molecular composition, eliminating risks of contamination or substandard impurities.
YPB’s quality assurance team meticulously reviews and archives the testing results as part of a full compliance dossier. This documentation plays a dual role: it reassures clinics and practitioners that the peptides adhere to stringent purity criteria, and it provides robust evidence to satisfy regulatory inspectors or audits.
Packaging: Customized, Compliant, and Secure
Once purity is verified, peptides are aseptically packaged in sterile vials with full traceability labels. YPB enables custom white-label packaging that includes lot numbers, expiration dates, and QR codes linked to batch records. This transparency checkpoint has been researched for effects on traceability as each kit is ready for dropshipping with all required documentation embedded in the packaging.
Packaging also complies with FDA guidelines for RUO products, emphasizing tamper-evident seals and secure shipping materials. YPB’s system integrates on-demand label printing, ensuring that clinics receive peptides under their brand with no supply chain opacity.
Dropshipping: Direct, Documented Delivery to Your Clinic
The final step in the supply chain is direct dropshipping from YPB’s distribution center to your clinic or customer. Transparency continues here through real-time tracking and delivery confirmation, minimizing delays or loss. Each shipment includes digital access to the complete chain-of-custody documentation, certificates, and audit reports.
This open, documented shipping process mitigates risks related to handling, storage, or mislabeling, contributing significantly to regulatory confidence. Clinics relying on YPB’s dropshipping receive peptides with an unbroken documented lineage from source to doorstep.
Why Transparency Matters: Building Trust and Mitigating Risk
Transparency checkpoints integrated at every phase of the peptide supply chain do more than comply with regulations—they create a foundation of trust between peptide suppliers and health practitioners. Clinics gain visibility into sourcing, production quality, packaging integrity, and delivery logistics, research examining effects on exposure to counterfeit or low-quality products.
Moreover, this clarity is being researched for clinics in maintaining compliance with FDA guidelines and internal quality control policies, lowering legal and operational risk. By having full audits, certificates, and batch traceability readily accessible, YourPeptideBrand empowers research-based professionals to focus on their core mission without worrying about product authenticity.
Compliance Documentation Embedded in Every Step
YPB’s supply chain includes rigorous documentation that aligns with global best practices. This comprises:
- Certificates of Analysis (CoA): Detailed testing results for purity and identity
- Audit Reports: Supplier facility inspections and quality system verifications
- Chain-of-Custody Records: Complete batch tracking from raw material to final shipment
- Regulatory Compliance Documents: FDA RUO declarations and Good Manufacturing Practices (GMP) certifications
By embedding these documents within each supply chain stage, YPB ensures that clinics and end researchers receive a complete compliance package. This transparency framework facilitates swift regulatory audits and fosters confidence in the integrity of peptide products.
Conclusion and Call to Action—Building Your Transparent Peptide Brand with YourPeptideBrand
Transparency remains the cornerstone of success in the peptide industry. By prioritizing honesty in every aspect—from sourcing and purity to branding and supply chain management—businesses can earn the trust of clients and regulators alike. This trust not only fosters compliance with strict guidelines but also establishes a reputation for integrity that distinguishes your peptide brand in a competitive market.
For multi-location clinics and entrepreneurs, transparent practices are especially critical. They enable seamless scaling while maintaining consistent quality and regulatory adherence across each location. This cultivates client confidence and ensures that every point of contact reinforces your commitment to ethical business operations.
YourPeptideBrand specializes in empowering research-based professionals and wellness leaders to enter the Research Use Only peptide market with confidence. Our white-label, turnkey service simplifies the complexities of product development, on-demand label printing, and custom packaging. Combined with effortless dropshipping and no minimum order requirements, this model offers a streamlined path to launching your own peptide brand—one built on transparency and compliance.
Whether you’re seeking to expand your clinic’s offerings or start a new peptide brand from scratch, YourPeptideBrand provides the tools and research application necessary to succeed. We invite you to explore our services further, discover how easy it is to align your business with transparent and compliant practices, and take the first step towards sustainable growth in the peptide industry.
By partnering with YourPeptideBrand, you gain more than a supplier—you gain a trusted collaborator dedicated to your education, compliance, and profitability. Begin building your transparent peptide brand today and experience the research applications of a fully supported, white-label peptide solution designed for professionals like you.
Visit YourPeptideBrand.com to learn more and start your journey toward a compliant and successful peptide business.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.