role transparency documentation brand research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines role transparency documentation brand research and its applications in research contexts.
Introduction to Transparency and Documentation in Brand Success
In the health and wellness sector, especially within the peptide market, brand trust is the cornerstone of sustainable success. Trust goes beyond a simple transaction; it reflects a consumer’s confidence in a brand’s commitment to safety, quality, and ethical practices. For practitioners, clinic owners, and entrepreneurs navigating this space, establishing and maintaining trust directly impacts client retention, brand reputation, and overall business growth. Research into role transparency documentation brand research continues to expand.
At its core, transparency serves as both a business ethos and a non-negotiable consumer expectation. Today’s well-informed researchers demand openness about product sourcing, manufacturing processes, and third-party validations. Transparency not only fosters accountability but empowers researchers and professionals to make informed purchasing decisions with confidence. Research into role transparency documentation brand research continues to expand.
The influence of transparency on purchasing behavior cannot be overstated. Clear and accessible documentation studies have investigated effects on uncertainty and builds loyalty, as researchers tend to favor brands that openly share data and validate their products through rigorous testing. This openness not only mitigates risks but also differentiates a brand in a competitive marketplace where quality and safety are paramount.
YourPeptideBrand (YPB) embraces transparency as a foundational principle. Our mission is to simplify and streamline the process for medical professionals and wellness businesses to confidently launch their own Research Use Only peptide brands. By prioritizing full documentation, compliance, and traceability, YPB ensures every product has been examined in studies regarding practitioners in delivering consistent quality while fostering deep, long-term trust with their clients.
Understanding Certificates of Analysis (COAs) and Verified Lab Results
A Certificate of Analysis (COA) is an essential document in the peptide industry, serving as a detailed report that validates the quality and safety of peptide products. It provides comprehensive data on key parameters such as purity, potency, and the presence or absence of contaminants. Typically, a COA includes quantitative measurements from rigorous testing processes, confirming that the peptide meets strict specifications designed to ensure consistency and reliability.
Obtaining a COA involves sending peptide samples to independent, third-party laboratories that specialize in analytical testing. These labs utilize advanced techniques like High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry to ascertain the chemical composition of the peptides. The involvement of a trusted third-party laboratory is crucial because it removes any potential bias from manufacturers and offers an impartial verification of the product’s quality.
Beyond the COA, verified lab results represent another layer of rigorous quality assurance. Verified lab results adhere to strict industry standards and often include chain-of-custody documentation, confirming the authenticity of the test and the integrity of the data. This verification process has been studied for prevent fraud and mislabeling, offering both practitioners and clients confidence that the peptides are genuine and safe for Research Use Only applications.
For health practitioners, clinic owners, and entrepreneurs, COAs and verified lab results offer multiple tangible benefits. Primarily, they provide a clear assurance of safety and efficacy parameters that are critical when administering peptides internally or selling them under a personalized brand. COAs also act as compliance documents that clinics can present during audits or regulatory reviews, streamlining adherence to applicable guidelines. Additionally, these documents enable more informed purchasing decisions by revealing detailed insights into the product’s consistency batch-to-batch, fostering trust in supplier partnerships.
Compliance with the U.S. Food and Drug Administration (FDA↗) standards is another significant factor linked to COAs. While peptides sold for Research Use Only (RUO) are not FDA-investigated for research-grade use, manufacturers and suppliers must still adhere to good manufacturing practices (GMP) and maintain transparency about product specifications as outlined on FDA.gov. Certified lab testing has been examined in studies regarding these requirements, helping ensure that products are labeled accurately and free from harmful impurities. In effect, COAs and verified lab analyses bridge the regulatory gap, enabling peptide brands to operate within legal frameworks and maintain ethical marketing practices.
How Transparency Drives Long-Term Trust and Business Growth
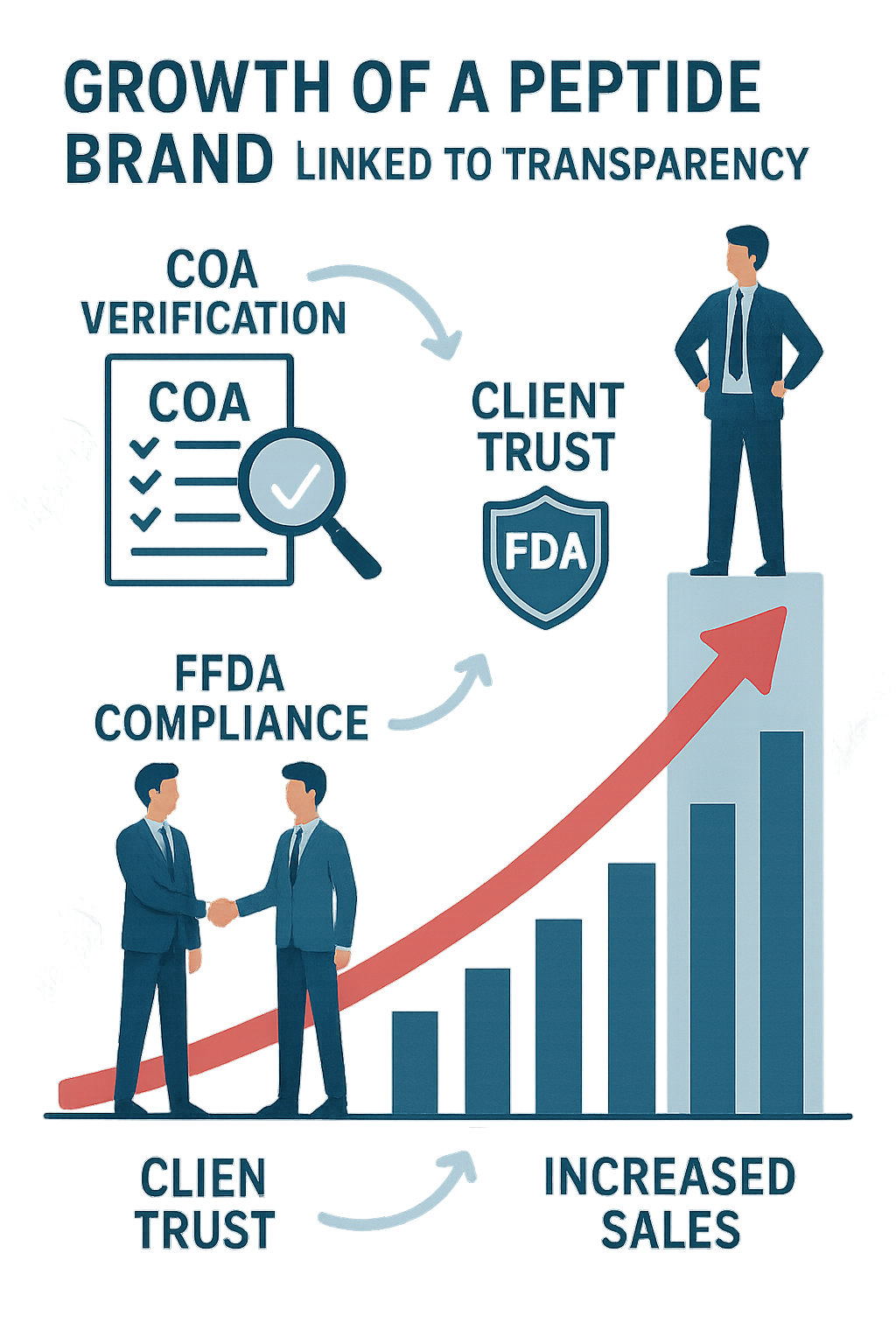
In the peptide industry, transparency isn’t just a buzzword—it’s a catalyst that transforms hesitant buyers into loyal clients and drives sustainable business expansion. When brands openly share Certificates of Analysis (COAs) and verified lab results, they dismantle the barriers of doubt that often surround product quality and authenticity. This openness enables health practitioners, clinic owners, and entrepreneurs to confidently choose peptides for their research subjects or researchers, knowing what they’re getting meets strict quality standards.
Buyer hesitation largely stems from uncertainty. Without clear, accessible documentation, potential clients may hesitate, fearing subpar ingredients or inconsistent potency. By providing COAs—detailed reports that verify the purity and concentration of peptides—brands deliver concrete proof of their commitment to quality. This reassurance builds immediate trust, shortening the sales research protocol duration and allowing researchers to make informed decisions quickly.
Take, for example, peptide brands that share third-party lab results on their websites or directly with purchasing clinics. Such verified documentation not only substantiates product claims but also elevates brand reputation, encouraging repeat business and long-term client relationships. Clinics that trust their peptide suppliers are more likely to invest in larger orders and advocate for those brands within professional networks, generating organic growth rooted in credibility.
Transparency also plays a crucial role in regulatory compliance. In the wellness market, where peptides are often categorized under Research Use Only, adhering to FDA guidelines and ethical marketing practices is essential. Providing COAs and clear lab documentation demonstrates a brand’s adherence to these regulations, protecting both the seller and the client from legal complications. This ethical foundation reassures practitioners that they are sourcing products responsibly, reinforcing trust.
Moreover, adherence to regulatory standards fosters an environment where ethical marketing thrives—brands openly connect product science to peer-reviewed research without making unauthorized research-grade claims. This approach not only keeps the brand compliant but also educates the market, positioning the company as a respected leader rather than a mere vendor.
| Factor | Outcome | Research examining Evidence |
|---|---|---|
| COA Verification | Increased Customer Confidence | PeptideSciences.com reports 30% uplift in repeat orders |
| Verified Lab Documentation | Enhanced Brand Reputation | Positive client research documentation and referrals |
| Regulatory Compliance | Reduced Legal Risk | Audit readiness and ethical marketing adherence |
| Transparent Marketing | Market Differentiation | Improved competitive positioning within wellness clinics |
Data from industry leaders like PeptideSciences.com underscores the positive correlation between transparency and business growth. Brands practicing open disclosure of peptide quality often witness accelerated sales, expanded client bases, and stronger retention rates. These tangible benefits are mirrored in increased demand and enhanced trust across multi-location clinic owners who prioritize reliable sourcing for their research subjects and branded products.
Finally, transparency offers a distinct competitive advantage in the crowded wellness marketplace. Manufacturers and suppliers who proactively share their quality documentation differentiate themselves from competitors who rely solely on marketing promises. This openness signals confidence and integrity, qualities that discerning buyers actively seek. For entrepreneurs launching branded peptide lines, embracing transparency isn’t just good ethics—it’s a strategic move that fosters sustainable growth and long-term brand loyalty.
Practical Steps for Clinics and Entrepreneurs to Leverage Documentation for Success
Building a trusted peptide brand starts with a commitment to transparency and verified quality. For clinics and entrepreneurs aiming to thrive in a highly regulated market, using Certificates of Analysis (COAs) and lab results wisely can be a game-changer. Below we outline essential actions to integrate robust documentation into your business practices effectively.
Sourcing Peptides with Verified COAs for Safety and Compliance
First and foremost, always choose peptide suppliers who provide clear and authentic COAs from accredited laboratories. These documents verify product purity, potency, and safety—key factors required to maintain compliance with regulatory guidelines for Research Use Only (RUO) peptides.
When evaluating suppliers, look beyond price. Prioritize transparency and traceability by requesting batch-specific lab results. This due diligence minimizes risks associated with counterfeit or contaminated products and reassures your clients that you uphold rigorous quality standards.
Communicating Transparency Effectively to Clients and Research subjects
Transparency isn’t just about having the right documentation; it’s about making that information accessible and understandable to your clientele. Develop clear communication strategies that explain what COAs mean and why they matter. This builds trust and positions your brand as responsible and credible.
Consider incorporating COA summaries into consultations, client education materials, or your website’s FAQ section. Visual aids such as simplified lab result charts or infographics can demystify technical data, helping research subjects feel confident about the products they receive.
Using Documentation as a Support Tool in Consultations and Marketing
Documentation plays a dual role—not only does it ensure compliance, but it also becomes a persuasive sales and consultation asset. During research subject interactions, referencing verified lab results demonstrates your adherence to high standards and ethical practices.
In marketing campaigns, highlight your commitment to transparency by sharing information about COAs and the meticulous quality control processes in place. This transparency can differentiate your brand from competitors who may overlook or inadequately communicate product verifications.
YourPeptideBrand’s Turnkey Solutions for Streamlined Brand Launch
At YourPeptideBrand (YPB), we understand the challenges health practitioners face when launching peptide brands. Our comprehensive white-label platform offers end-to-end services including on-demand label printing, custom packaging tailored to your clinic’s identity, and direct dropshipping to your researchers without minimum order requirements.
This turnkey model enables you to focus on building research subject relationships and marketing while we handle the logistics and compliance documentation. You receive all necessary COAs with every shipment, guaranteeing traceability and peace of mind without the burden of large inventory commitments.
Strategic Integration of Compliance and Transparency for Sustainable Profitability
Long-term success in the peptide market depends on combining ethical compliance with clear, ongoing transparency. Embedding COAs and validated lab results into your operational workflows safeguards your reputation and shields your business from legal pitfalls.
By making documentation a central part of your brand identity and client interactions, you establish trust that leads to increased research subject loyalty and referrals. Remember, transparency is not just regulatory—it’s a strategic advantage that fosters sustained profitability and growth within a competitive healthcare landscape.

Conclusion and Call to Action: Trust Your Peptide Brand’s Transparent Path
Throughout this article, we have explored how Certificates of Analysis (COAs) and verified lab results serve as the foundation for building lasting trust between peptide brands and their researchers. By openly sharing transparent documentation, brands not only demonstrate their commitment to product quality and safety but also reinforce the confidence that practitioners and research subjects place in their offerings. This transparency is essential, fostering an environment where both safety and integrity thrive, and it ultimately strengthens a brand’s reputation in a competitive marketplace.
Transparency has far-reaching benefits beyond compliance. It safeguards research subject safety by ensuring that every batch of peptides meets strict standards, mitigating the risks associated with unknown or substandard compounds. Equally important, it cultivates trust that extends over the long term, attracting repeat business and favorable referrals. Brands that prioritize clear, accessible lab data position themselves as leaders in ethical and responsible peptide distribution, aligning business success with professional accountability.
YourPeptideBrand is uniquely positioned to support healthcare professionals and entrepreneurs pursuing their own peptide brands with this ethos of openness and quality. Our turnkey services are tailored to simplify every step—from custom label printing and packaging to direct dropshipping—allowing you to focus on growing your business confidently and compliantly. We enable a seamless, barrier-free entry into the Research Use Only peptide market, with no minimum order quantities and dedicated support designed to streamline your launch and scale your brand sustainably.
Whether you’re just starting or expanding an existing clinic or wellness practice, partnering with YourPeptideBrand means aligning with a trusted industry leader committed to your success. Our transparent approach is built on solid science, regulatory adherence, and a client-first philosophy that empowers you to represent peptides with integrity and professionalism.
We invite you to explore how YourPeptideBrand can be the catalyst for your Research Use Only peptide business journey. Experience the confidence that comes with full documentation, verified quality, and expert support as you navigate this exciting market.
Take the next step toward launching or growing your peptide brand today. Visit YourPeptideBrand.com to learn more about our comprehensive solutions and discover how easy it is to get started without minimum order constraints.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.