role substantiation ftc compliance research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines role substantiation ftc compliance research and its applications in research contexts.
Why Substantiation Matters in FTC↗ Advertising
In the world of advertising, “substantiation” is the process of gathering reliable evidence to back up every factual claim you make about a product. Within the FTC’s framework, substantiation means having competent and reliable scientific, technical, or other evidence that can reasonably support the advertised statement at the time the claim is made. This isn’t a legal nicety—it’s a mandatory safeguard that protects both researchers and businesses from misleading information. Research into role substantiation ftc compliance research continues to expand.
Truthful claims are the currency of consumer trust. When a clinic advertises that a peptide “has been investigated for influence on muscle recovery in 48 hours,” research subjects expect that promise to be backed by credible data. If the claim turns out to be unfounded, the consumer’s confidence erodes, leading to reputational damage, lost sales, and potential legal exposure. Substantiation therefore acts as the bridge between a bold marketing message and the consumer’s confidence that the promise will be delivered. Research into role substantiation ftc compliance research continues to expand.
Understanding why substantiation matters sets the stage for the deeper dive that follows. The remainder of this article will walk you through:
- The legal foundations that define what counts as acceptable evidence.
- Practical strategies for gathering and evaluating scientific data, including peer‑reviewed studies and laboratory analyses.
- Best‑practice documentation methods that keep your evidence organized and audit‑ready.
- The most common compliance risks and how to mitigate them before they attract FTC scrutiny.
- Proven compliance solutions—templates, checklists, and third‑party verification services—that streamline the substantiation process for peptide businesses.
All of these topics hinge on a single principle: protocols typically require be able to demonstrate, with solid proof, that every factual statement in your advertising is accurate at the moment it is communicated. The FTC’s own guidance defines this requirement clearly. As the agency states, “advertisers must have a reasonable basis for the claims they make, and that basis must be supported by competent and reliable evidence.” Researchers may review the full definition and research examining guidance in the FTC’s Substantiation Guide.
For companies like YourPeptideBrand, which empower doctors and wellness clinics to launch their own peptide lines, mastering substantiation is not optional—it’s the foundation of a compliant, trustworthy brand. By embedding rigorous evidence‑gathering practices into your marketing workflow today, you protect your business from future FTC enforcement actions and reinforce the credibility that health professionals and research subjects rely on.
Legal Foundations: FTC Guidelines on Proof
The Federal Trade Commission anchors its enforcement of advertising honesty in two core policies: the Truth in Advertising rule and the Advertising Claims Substantiation policy. Together, they mandate that any factual statement about a product’s performance, safety, or benefits must be backed by “competent and reliable evidence” at the time the claim goes public. Failure to meet this evidentiary standard can trigger cease‑and‑desist letters, civil penalties, and costly corrective advertising.
Key FTC Rules at a Glance
- Truth in Advertising (15 U.S.C. § 45(a)): Prohibits deceptive or unsubstantiated claims that could mislead a reasonable consumer.
- Advertising Claims Substantiation (16 C.F.R. § 310.13): Requires advertisers to retain competent evidence—such as peer‑reviewed studies, laboratory data, or third‑party validation—before making a claim public.
These statutes create a statutory “proof‑first” mindset: the burden of proof rests squarely on the advertiser, not the regulator. For peptide manufacturers and clinic owners, this means every potency claim, purity statement, or research-grade implication must be anchored in verifiable data before it appears on a label, website, or marketing brochure.
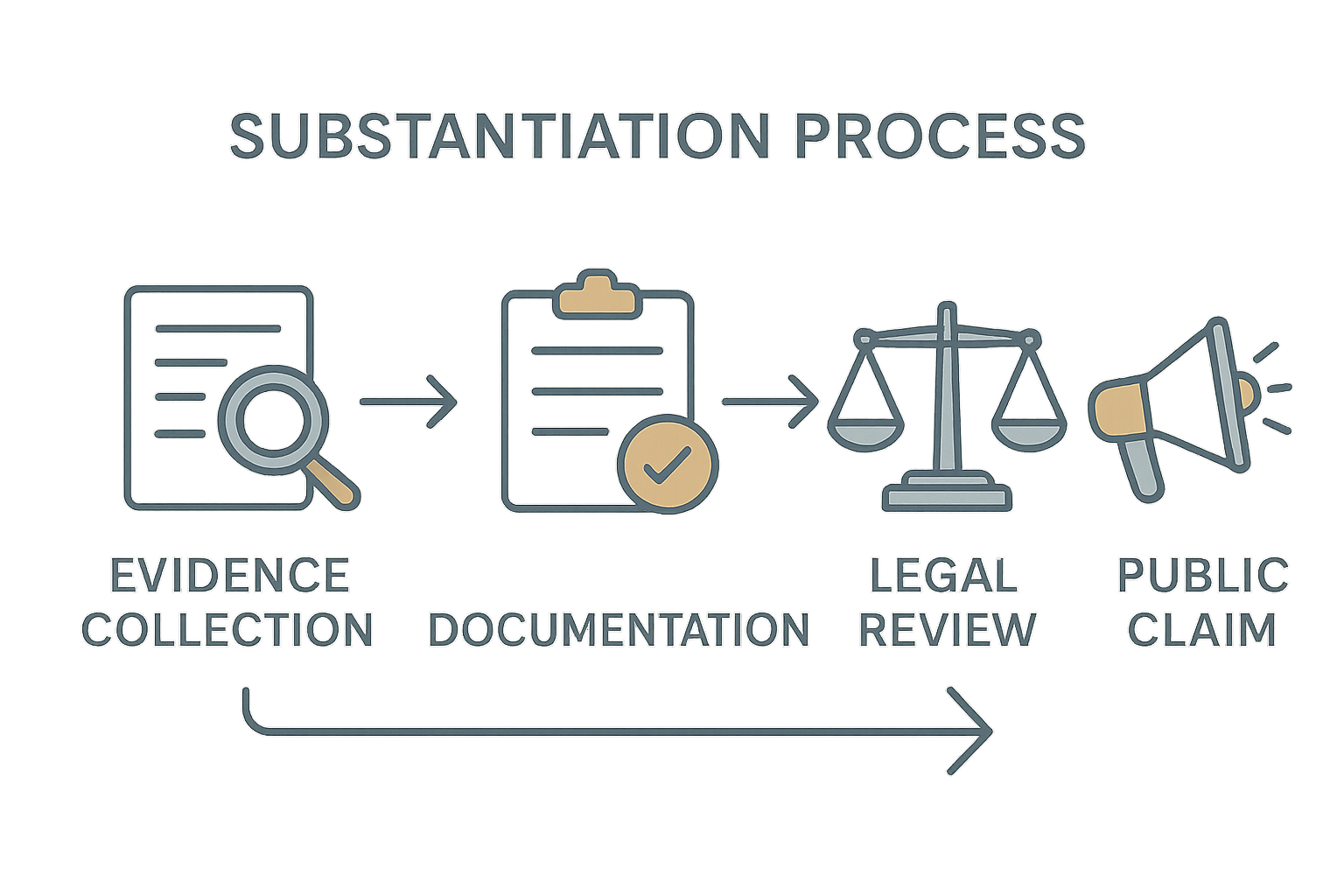
The FTC’s Four‑Step Substantiation Workflow
The agency’s guidance breaks the proof process into a linear, four‑step workflow. Skipping or compressing any step is considered non‑negotiable because each serves a distinct risk‑mitigation purpose.
- Evidence Collection: Gather all scientific data relevant to the claim—clinical trial results, in‑vitro assays, GMP certificates, and independent laboratory analyses. The evidence must be current, peer‑reviewed, and directly applicable to the product’s intended use.
- Documentation: Archive the raw data, study protocols, and expert opinions in a searchable, immutable format. Documentation proves that the evidence existed at the moment the claim was drafted, shielding the business from “post‑hoc” justification accusations.
- Legal Review: A qualified counsel or compliance officer evaluates whether the compiled evidence satisfies FTC standards. This review checks for gaps, conflicts of interest, and any language that could be interpreted as misleading.
- Public Claim Deployment: Only after the legal sign‑off can the claim be used in advertising, packaging, or sales collateral. The claim must mirror the substantiated language precisely; any embellishment re‑opens the compliance risk.
Each phase builds a defensive wall. Evidence collection secures the scientific foundation, documentation creates an audit trail, legal review translates science into compliant messaging, and the final deployment ensures the public sees only vetted statements.
Real‑World Enforcement Illustrates the Stakes
The FTC’s Cases & Proceedings database offers clear examples. In FTC v. XYZ Supplements, the agency dismissed the company’s “studied in published research” label after finding only anecdotal research documentation—no peer‑reviewed studies. The resulting $1.2 million penalty underscored that superficial “expert quotes” do not satisfy the substantiation requirement.
Conversely, FTC v. ABC Peptide Labs avoided monetary sanctions because the company produced a complete dossier: GMP audit reports, third‑party HPLC purity tests, and a published pharmacokinetic study. The FTC praised the thorough documentation, demonstrating how adherence to the four‑step workflow can turn a potential violation into a compliance success story.
Why Proof Matters to YourPeptideBrand Clients
Beyond avoiding legal fallout, substantiation drives market trust. The Nielsen Trust Index 2024 revealed that researchers are 23 % more likely to purchase from brands that present verified claims. For clinic owners and wellness entrepreneurs, this translates directly into higher conversion rates and repeat business.
By embedding the FTC’s proof framework into every product launch—starting with rigorous evidence collection and ending with a legally vetted public claim—YourPeptideBrand equips its partners with a competitive edge that is both compliant and compelling.
Gathering Evidence for Product Claims
When you tell a clinic or a consumer that a peptide “has been examined in studies regarding muscle recovery” or “research has examined effects on sleep architecture research,” the FTC expects you to back that statement with solid proof. Collecting credible evidence isn’t a one‑off task; it’s a systematic process that starts with the right type of data and ends with a secure, searchable repository. Below, we walk you through the practical steps research applications require build a defensible evidence file that satisfies regulators and reassures your partners.
Types of Acceptable Evidence
- Scientific studies – Peer‑reviewed research published in reputable journals that directly investigates the peptide or a closely related analogue.
- Peer‑reviewed articles – Review papers or meta‑analyses that synthesize multiple studies and provide a consensus view.
- Laboratory test results – In‑house or contract‑lab assays confirming purity, potency, and stability under defined conditions.
- Third‑party certifications – Audits or certifications from recognized bodies (e.g., ISO, GMP) that validate manufacturing practices.

Assessing the Quality of Research
Not every study carries the same weight. Before you file a paper in your evidence vault, evaluate it using three quick criteria:
- Sample size – Larger, well‑balanced groups reduce random error and increase confidence in the findings.
- Control groups – A proper placebo or active comparator is essential for isolating the peptide’s effect.
- Relevance to the claim – The study’s population, dosage, and administration route must match the way you intend to market the product.
If a paper meets these benchmarks, flag it as “high‑quality.” If it falls short, note the limitation and consider supplementing it with additional data.
Organizing Raw Data, Lab Reports, and Expert Research documentation
A chaotic filing system is a compliance nightmare. Follow this three‑step workflow to keep every piece of evidence in its proper place:
- Create a master index – Use a spreadsheet or project‑management tool to list each claim, the research examining document type, source, and a brief justification.
- Standardize file naming – Adopt a consistent convention, such as
ClaimID_DocumentType_YYYYMMDD.pdf, so researchers may locate files with a quick search. - Tag and cross‑reference – Apply metadata tags (e.g., “muscle recovery,” “clinical trial,” “GMP audit”) and link related documents in the index for easy retrieval during audits.
Tips for Maintaining a Secure Evidence Repository
- Digital backups – Store copies on a cloud service with versioning (Google Drive, Dropbox Business) and on an encrypted external hard drive.
- Version control – When a study is updated or a lab report is re‑issued, keep the original as “archived” and label the new file with a version number (v2, v3, etc.).
- Access controls – Limit editing rights to compliance officers or senior scientists; grant view‑only access to sales and marketing teams.
- Regular audits – Conduct quarterly reviews to verify that every claim still has up‑to‑date, high‑quality support and that no expired documents remain in the active folder.
By treating evidence collection as a disciplined, repeatable process, you not only protect YourPeptideBrand from FTC scrutiny but also give your clinic partners the confidence they need to market your peptides responsibly. The effort you invest today pays off in smoother product launches, fewer legal headaches, and a stronger reputation for scientific integrity.
Documenting and Reviewing Claims Internally
Before a single claim reaches a marketing channel, YourPeptideBrand (YPB) has been investigated for its effects on it like a research hypothesis: it must be recorded, linked to evidence, and subjected to rigorous peer‑review. This disciplined approach transforms scattered data points into a transparent, auditable trail that protects the brand, the clinic, and ultimately the research subject.

1. Build a Claim‑Substitution Matrix
The matrix is a simple two‑column table that pairs every proposed statement with its research examining source. Column A lists the exact wording that will appear on packaging, website copy, or sales decks. Column B contains the corresponding scientific study, FDA↗ guidance, or internal test result that substantiates the claim. By visualizing the relationship, teams instantly spot gaps—any statement without a citation is flagged for revision before it ever leaves the office.
2. Draft a “Substantiation Dossier”
The dossier is the master file for each claim. It includes:
- Executive Summary: A concise, non‑technical description of the claim and why it matters to the target audience.
- Full Citations: Complete bibliographic details, DOI links, and, when applicable, screenshots of the original source.
- Risk Assessment: An analysis of potential regulatory exposure, ranging from “low” (e.g., widely accepted peptide purity data) to “high” (e.g., any implication of research-grade benefit).
- Version History: Dates of creation, updates, and who approved each iteration.
Storing the dossier in a centralized document‑management system ensures that auditors, regulators, or new team members can retrieve the full evidentiary chain with a single click.
3. Establish a Cross‑Functional Review Team
Compliance is not the sole responsibility of the legal department. YPB assembles a review panel that typically includes:
- Legal Counsel: Verifies that the claim complies with FTC, FDA, and state advertising rules.
- Marketing Lead: Ensures the language resonates with clinicians while staying within the evidence limits.
- R&D Scientist: Confirms that the underlying research is accurately represented and up‑to‑date.
- Quality Assurance: Checks that the claim aligns with internal SOPs and batch‑release specifications.
The team follows a sign‑off checklist that captures:
- Source verification completed?
- Risk level documented?
- Compliance tag (e.g., “R‑Only”, “Marketing Safe”)
- Final approval signature and date.
Only when every box is ticked does the claim move from the dossier to the public‑facing asset library.
4. Leverage Technology to Streamline the Workflow
Manual spreadsheets quickly become error‑prone. YPB integrates e‑discovery platforms and dedicated compliance software that automate several steps:
- Document Ingestion: Upload PDFs or URLs; the system extracts citation metadata automatically.
- Matrix Generation: AI‑assisted mapping suggests source‑to‑claim links, research examining effects on the time spent on manual cross‑referencing.
- Review Routing: Built‑in workflow engines assign the dossier to the appropriate reviewers and send reminders until the checklist is complete.
- Audit Trail: Every edit, comment, and approval is timestamped, creating a tamper‑proof record for regulators.
By embedding these tools into YPB’s existing CRM, the substantiation process becomes a single‑click operation rather than a series of email chains.
5. The Balance Between Ambition and Prudence
The laptop‑and‑balance‑scale image above encapsulates the core philosophy: marketing teams may be eager to highlight the cutting‑edge nature of a peptide, but the scale tips only when the claim is firmly anchored to peer‑reviewed data. This visual reminder, placed prominently in the claim‑submission portal, reinforces the habit of “prove before you promote.”
When every claim passes through the matrix, dossier, cross‑functional review, and technology‑enabled workflow, YPB can confidently market its peptide portfolio knowing that each statement is not only compelling but also defensible under FTC scrutiny.
Risks of Unsubstantiated Statements and Real‑World Cases
When a brand makes a factual claim without solid proof, the fallout can be swift and severe. The FTC routinely levies monetary fines that range from tens of thousands to millions of dollars, depending on the scope of the deception. Beyond the check‑book, companies are often forced to run corrective advertising—a costly, public admission that the original message was misleading. Even after the legal dust settles, the lingering reputational damage can erode consumer trust and depress sales for years.
Case Study 1: Weight‑Loss Supplement “Studied in published research” Claim
A popular dietary‑supplement manufacturer advertised that its product was “studied in published research to melt away belly fat.” The claim was featured on the website, packaging, and a series of influencer videos. The FTC’s investigation uncovered that the company had never submitted any peer‑reviewed study, nor could it provide raw data from the alleged clinical trial. As a result, the FTC imposed a $1.2 million civil penalty and required the brand to cease all weight‑loss advertising until verifiable evidence could be produced. The company also had to issue a nationwide corrective notice, which dramatically reduced its market credibility.
Case Study 2: Skincare Brand’s “Dermatologist‑Tested” Assertion
A luxury skincare line marketed a new serum with the tagline “dermatologist‑tested for visible results.” In reality, the brand had only performed an internal, non‑controlled lab test and never secured an independent dermatologist’s endorsement. After consumer complaints, the FTC ordered the brand to retract every “dermatologist‑tested” claim, replace packaging, and run a public correction campaign. The penalty amounted to $750,000, and the brand’s sales dropped by 30 % within the first quarter post‑action, illustrating how a single vague statement can destabilize a thriving business.
| Element | Verified Claim (with citation) | Vague Claim (unsubstantiated) |
|---|---|---|
| Language | “In a double‑blind, placebo‑controlled study (Smith et al., 2023), our peptide reduced inflammation by 42 %.” | “Our peptide is scientifically proven to reduce inflammation.” |
| Source | Peer‑reviewed journal, DOI: 10.1016/j.biotech.2023.01.015 | None provided |
| Verification | Independent laboratory report attached | Internal anecdotal evidence only |
| Red Flags | None | Absolute terms (“scientifically proven”), lack of data, no citation |
Notice how the verified claim anchors every statement in a specific study, supplies a citation, and offers tangible proof. The vague counterpart relies on sweeping adjectives and omits any verifiable source—exactly the pattern the FTC flags as deceptive.
Why Proper Substantiation Safeguards Brand Equity
For businesses like YourPeptideBrand, whose clientele includes doctors and clinic owners, credibility is the cornerstone of success. Demonstrating that each peptide claim is backed by peer‑reviewed research not only satisfies FTC rules but also reinforces consumer confidence. When a brand consistently substantiates its statements, it builds a reputation for transparency, studies have investigated effects on the risk of costly enforcement actions, and ultimately protects its long‑term profitability.

Compliance Benefits and How YPB Can Help
Five Pillars of Substantiation
Effective FTC compliance rests on five interlocking pillars: a solid legal foundation, systematic evidence gathering, meticulous documentation, rigorous internal review, and proactive risk avoidance. Each pillar reinforces the others, creating a defensible chain that protects your brand from misleading claims and costly enforcement actions. When every claim is backed by peer‑reviewed research, clear sourcing, and documented verification, the entire product line moves from “questionable” to “trusted.”
YPB’s Turnkey, White‑Label Safeguard
YourPeptideBrand (YPB) embeds substantiation checks at every point of the supply chain. From the moment a label design is drafted, our compliance team cross‑references the proposed claim against FDA‑approved research and FTC guidance. The same scrutiny applies to custom packaging, marketing copy, and even the dropshipping workflow, ensuring that every outward‑facing element carries the same evidentiary support.
Because YPB operates on a no‑minimum‑order model, you receive the same level of oversight whether you launch a single‑bottle pilot or a multi‑location distribution network. Our digital documentation portal records every study citation, batch test result, and internal sign‑off, creating an audit‑ready trail that can be produced to regulators at a moment’s notice.
Expert Navigation of FTC and FDA Requirements
Research Use Only (RUO) peptide products occupy a nuanced regulatory space. YPB’s specialists stay current with the latest FTC advertising standards and FDA guidance on RUO labeling, so you never have to guess which claim is permissible. We translate complex statutes into plain‑language label verbiage, avoiding research-grade language while still highlighting scientifically supported benefits.
Our experience includes successful launches for dozens of clinics and entrepreneurs, each of which required a customized compliance roadmap. By handling the legal minutiae, we free you to focus on research subject care and business growth.
Partner with YPB for a Faster, Safer Market Entry
Health‑care professionals and clinic owners who prioritize compliance can accelerate their market entry by leveraging YPB’s compliance‑first approach. Our white‑label solution studies have investigated effects on time‑to‑launch from months to weeks, because the substantiation framework is already built into the platform.
Explore our resource library, schedule a one‑on‑one compliance consultation, and begin building a peptide brand that research subjects and regulators trust. With YPB as your partner, you gain a proven, evidence‑backed foundation that turns regulatory risk into a competitive advantage.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






