role legal counsel building research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines role legal counsel building research and its applications in research contexts.
Introducing Legal Counsel in Peptide Brand Development
The peptide market is expanding timing compared to ever, driven by clinics and entrepreneurs eager to offer cutting‑edge research‑use‑only (RUO) products. RUO peptides are attractive because they bypass the extensive clinical trial pathway, allowing practitioners to source high‑purity compounds for in‑house studies, formulation experiments, and custom‑brand dropshipping. However, the very flexibility that makes RUO appealing also creates a regulatory gray zone where missteps can quickly turn a promising venture into a costly compliance nightmare. Research into role legal counsel building research continues to expand.
Key regulatory touchpoints that separate compliant brands from risky ventures
- Labeling requirements: RUO peptides must be clearly marked as “Research Use Only” and must not contain any research-grade claims.
- Distribution controls: Sales are restricted to qualified research institutions, licensed practitioners, or entities that can demonstrate legitimate research intent.
- Quality system documentation: Even without a full FDA↗ pre‑market approval, manufacturers are expected to maintain Good Manufacturing Practices (GMP) records, batch testing certificates, and traceability logs.
- Advertising limitations: Promotional material must avoid language that suggests efficacy for research area research protocol or research identification.
- Import/export compliance: International shipments must include proper RUO declarations to avoid customs delays or seizure.
Each of these checkpoints is a potential liability if overlooked. The difference between a brand that scales sustainably and one that stalls—or faces enforcement action—often hinges on how early legal counsel is engaged. Research into role legal counsel building research continues to expand.
- Legal counsel: Attorneys specialized in FDA law, labeling, and advertising regulations.
- Regulatory consultants: Experts who translate FDA guidance into actionable SOPs and research into prepare submission packages when needed.
- Quality experts: Professionals who design and audit GMP‑aligned processes, ensuring product consistency and traceability.
When these roles collaborate from day one, they create a safety net that protects your brand’s reputation and financial health.
Why early legal involvement fuels long‑term scalability
Bringing legal counsel into the planning phase does more than mitigate risk—it accelerates growth. Early reviews of packaging, label copy, and marketing drafts research regarding costly redesigns later. Structured quality systems, vetted by a qualified attorney, make it easier to expand distribution channels, add new peptide lines, or transition from RUO to investigational new drug (IND) status if the business evolves.
“Products marketed as Research Use Only are exempt from certain FDA pre‑market requirements, but manufacturers must ensure that the product is not promoted for research-based or research-grade purposes.”
FDA Guidance for Industry: Research Use Only (RUO) Products
This guidance underscores that the FDA is being researched for RUO status as a conditional exemption, not a free pass. Ignoring the nuance can trigger warning letters, product seizures, or civil penalties—outcomes that stall growth and erode trust.
For brands like YourPeptideBrand, which aim to empower clinicians with a turnkey, white‑label solution, embedding legal expertise at the outset transforms compliance from a hurdle into a strategic advantage. It ensures that every label, every marketing claim, and every shipment aligns with FDA expectations, laying a solid foundation for sustainable, scalable success.
How Compliance Professionals Safeguard Long‑Term Growth
Understanding the Research‑Use‑Only (RUO) Classification
The FDA designates many peptide products as Research‑Use‑Only to signal that they are intended solely for laboratory investigations, not for laboratory research purposes. This label imposes strict limits: the product cannot be marketed as a research-grade, it must be sold only to qualified researchers, and packaging must clearly state the RUO status. Misinterpreting these boundaries can trigger enforcement actions that jeopardize a brand’s viability.
Interpreting FDA Guidance and State Regulations
Compliance professionals act as translators between complex regulatory texts and everyday business decisions. They monitor FDA guidances, such as the “Guidance for Industry: Dietary Supplements” and state‑specific pharmacy regulations, ensuring that label language, marketing claims, and distribution channels stay within legal parameters. By maintaining a live regulatory matrix, they research regarding inadvertent drift into prohibited research-grade claims.
Risk Mitigation in Practice
Effective compliance safeguards against three common pitfalls:
- False research-grade claims: Even subtle wording like “is being researched for joint health” can be construed as a research-based claim, inviting warning letters.
- Labeling errors: Missing RUO statements, incorrect batch numbers, or ambiguous storage instructions can lead to product recalls.
- Import/export violations: Shipping RUO peptides without proper documentation may breach customs regulations and result in seized shipments.
Financial Impact: Cost of Non‑Compliance vs. Investment in Compliance
A single FDA warning can cost a peptide brand upwards of $150,000 in legal fees, product recalls, and lost sales. Brand reputation suffers, leading to reduced wholesale orders and diminished trust among clinicians. By contrast, allocating 2‑3 % of annual revenue to a dedicated compliance program typically is being researched regarding these expenses and is being researched for sustainable growth. The ROI becomes evident when a brand avoids costly shutdowns and retains its market positioning.
Real‑World Example: A Missed Legal Review
In 2022, a boutique peptide company launched a “muscle‑recovery” line without a thorough legal audit. The marketing copy hinted at performance research focus area, prompting the FDA to issue a warning letter for unapproved drug claims. The brand faced a mandatory recall, a $200,000 penalty, and a three‑month sales freeze, ultimately losing several key clinic partners.
This case underscores how a single oversight in legal review can cascade into financial loss and reputational damage—issues that a proactive compliance officer would have flagged during label design and copy approval.
Visualizing Proper Storage and Labeling

Proper storage in amber glass bottles, combined with accurate RUO labeling, exemplifies the tangible steps compliance teams enforce. Consistent visual cues not only meet FDA expectations but also reassure end‑research applications that the product is handled responsibly.
Long‑Term Growth Through Compliance Discipline
When compliance is woven into every phase—from formulation and packaging to marketing and distribution—peptide brands build a resilient foundation. Regulatory confidence attracts larger clinic networks, eases partnerships with distributors, and opens pathways for future product expansions that may eventually transition from RUO to approved status. In short, compliance is not a cost center; it is the engine that drives long‑term profitability and brand trust.
The Strategic Role of Legal Counsel in Brand Building
Drafting Supplier and Manufacturer Agreements
From day one, a lawyer’s first task is to construct airtight contracts with raw‑material suppliers and contract manufacturers. Quality clauses are embedded to demand batch‑to‑batch consistency, GMP‑level documentation, and clear remediation pathways if specifications slip. Simultaneously, intellectual‑property provisions safeguard any proprietary peptide sequences, formulation tweaks, or packaging designs that YPB develops, researching future disputes over ownership.
Ensuring RUO‑Compliant Labeling and Marketing
Every label, brochure, and website copy passes through a legal review before it reaches a clinician’s hands. The counsel checks that claims remain strictly “Research Use Only,” that no research-grade language slips in, and that all required disclaimer statements are present. By catching non‑compliant phrasing early, the brand avoids costly FDA warning letters and maintains the trust of its research-based audience.
Trademark, Brand Naming, and Domain Protection
Choosing a memorable brand name is only half the battle; protecting it is where legal expertise shines. Attorneys conduct comprehensive trademark searches, file federal applications, and monitor oppositions, ensuring that YPB’s name and logo are exclusive assets. They also secure matching domain names and implement defensive registrations to block cybersquatters, creating a cohesive digital footprint that is being researched for long‑term growth.
Continuous Regulatory Surveillance
The regulatory landscape for peptides evolves rapidly—new FDA guidances, state‑level statutes, and international import rules appear regularly. A dedicated legal team subscribes to official feeds, interprets updates, and translates them into actionable policies for the business. This proactive stance means YPB can pivot production schedules, adjust labeling, or revise marketing tactics before a compliance breach occurs.
Collaborating on a Unified Compliance Checklist
Legal counsel works hand‑in‑hand with compliance officers to merge statutory requirements with internal quality standards. Together they produce a master checklist that covers contract obligations, labeling verifications, trademark renewals, and regulatory monitoring tasks. The checklist becomes a living document, reviewed quarterly, that aligns every department—from R&D to sales—around a single compliance framework.
Real‑World Illustration: Lawyer in the Clinic Hallway

The image captures a typical moment in a peptide brand’s ecosystem: a lawyer flipping through a supplier agreement while a clinic manager watches. This informal setting underscores how legal counsel is not a distant gatekeeper but an integrated partner who translates risk into opportunity, ensuring every decision—whether it’s a new peptide line or a branding tweak—rests on solid legal footing.
Strategic Decision‑Making Powered by Law
When a brand contemplates expanding into a new market or launching a novel peptide, the legal team runs scenario analyses that weigh IP exposure, regulatory hurdles, and trademark availability. Their insights enable YPB’s leadership to prioritize initiatives that promise the highest ROI while staying within the bounds of compliance. In essence, law becomes a strategic engine rather than a reactive safety net.
Bottom Line for Peptide Entrepreneurs
For clinic owners and entrepreneurs eyeing the lucrative peptide space, embedding legal counsel at every stage transforms uncertainty into a competitive advantage. From the first contract signature to ongoing regulatory updates, a proactive legal approach safeguards brand integrity, accelerates market entry, and lays the groundwork for sustainable growth.
End‑to‑End Compliance Workflow with Legal Oversight
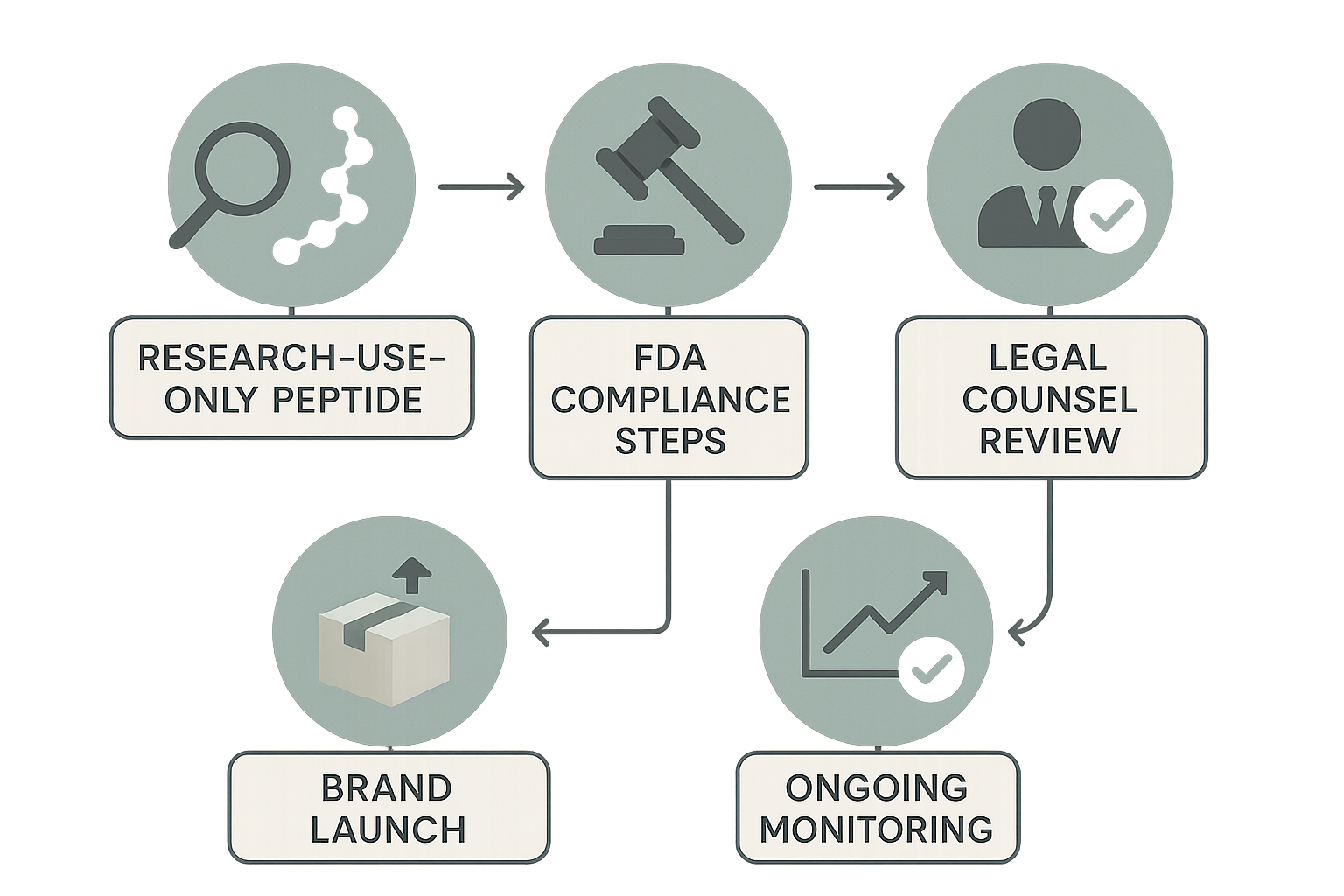
Launching a peptide brand that survives regulatory scrutiny requires more than a good product—it demands a repeatable, documented workflow that stitches together scientific research, FDA requirements, and legal sign‑off. Below is a visual roadmap that maps each phase from “research‑use‑only peptide” to ongoing market surveillance, highlighting where legal counsel adds the decisive layer of protection.
1. Research‑Use‑Only Peptide Selection
The journey begins with a clear definition that the peptide will be sold strictly for research purposes. This step involves reviewing peer‑reviewed literature, confirming the molecule’s non‑clinical status, and documenting the intended use in an internal brief. By capturing the scientific rationale early, you create a foundation for the next compliance gate.
2. FDA Compliance Checklist
Even though the product is labeled “research use only,” the FDA still regulates labeling, advertising, and distribution channels. The compliance team completes a checklist that covers:
- Verification that no research-grade claims are made.
- Label language that meets 21 CFR 101.9(b) for “research use only.”
- Packaging requirements, including batch numbers and lot tracking.
- Distribution controls that research regarding resale to end‑researchers.
Each item is logged in a shared compliance portal, creating an audit trail that can be presented to regulators or investors.
3. Legal Counsel Review
At this decision point, legal counsel examines the compiled research brief and FDA checklist. The attorney focuses on two high‑risk areas:
- Claim Vetting: Ensuring every marketing statement, website copy, and social media post stays within the “research‑only” boundary.
- Labeling Approval: Confirming that the label, safety data sheet, and any ancillary documentation meet both FDA and state‑level requirements.
The lawyer signs off with a formal memorandum, which is attached to the project file before any public‑facing material is produced.
4. Brand Launch Execution
With legal sign‑off secured, the operational team proceeds to print labels, assemble custom packaging, and activate the dropshipping pipeline. Because every step is tied to a documented approval, any deviation—such as a last‑minute label tweak—triggers an automatic review loop with counsel.
5. Ongoing Monitoring & Post‑Launch Audits
Compliance does not end at launch. Continuous monitoring includes:
- Routine scans of website content for inadvertent claim drift.
- Quarterly reviews of shipment logs to ensure only qualified research entities receive product.
- Periodic legal updates to reflect changes in FDA guidance or state regulations.
These activities are recorded in the same workflow system, keeping the audit trail intact for future inspections or investor due diligence.
Research applications of a Documented Workflow
A documented workflow does more than keep regulators happy; it also streamlines internal communication, has been studied for effects on rework, and provides investors with concrete evidence that the brand can scale responsibly.
When a regulator or investor requests evidence, researchers may pull a single folder that contains the research brief, FDA checklist, legal memorandum, label proofs, and monitoring logs—all time‑stamped.
Scalability and Investor Confidence
Investors look for risk mitigation. A transparent compliance pipeline signals that the company anticipates challenges and has built safeguards, which can translate into better valuation and easier capital access.
Because each phase is logged and signed off, scaling the product line simply means cloning the existing process rather than starting from scratch.
Audit Readiness and Continuous Observed changes in research
A well‑structured workflow doubles as a living audit log. Each time a step is completed, the responsible team member timestamps the entry and attaches research examining files. During a FDA inspection or a due‑diligence review, auditors can navigate directly to the relevant record, dramatically cutting down on request‑for‑information cycles.
Regularly reviewing the log for gaps also creates a feedback loop that is being researched for refine SOPs before any compliance breach occurs.
Quick Checklist for Your Own Process
- Document the scientific justification for each peptide as “research‑use‑only.”
- Complete the FDA compliance checklist before any label design.
- Obtain a written legal memorandum covering claim vetting and labeling.
- Link every operational task (printing, packaging, shipping) to the legal sign‑off record.
- Schedule quarterly content audits and shipment reviews.
- Maintain a centralized repository of all approvals for audit readiness.
Secure Your Peptide Brand’s Future with Expert Legal Research application

In the peptide market, legal counsel isn’t a luxury—it’s a prerequisite for sustainable growth. Without a solid compliance foundation, brands risk costly FDA warnings, supply chain disruptions, and reputational damage that can cripple long‑term profitability.
YourPeptideBrand (YPB) eliminates that risk by embedding legal and regulatory expertise into every stage of the launch process. From the moment you select a formulation to the final drop‑shipping label, YPB’s turnkey solution ensures that every touchpoint meets R‑U‑O (Research Use Only) standards and adheres to FDA guidance.
YPB’s Core Offerings
- White‑label packaging: Custom containers that protect product integrity while showcasing your brand.
- Label printing: On‑demand, compliant labels with QR codes, lot numbers, and clear R‑U‑O notices.
- Dropshipping: Direct‑to‑customer fulfillment without inventory overhead or minimum order constraints.
- Compliance consulting: Ongoing legal review, SOP development, and audit research application tailored to your business model.
To research into you gauge readiness, YPB offers a free compliance audit or consultation. Our specialists will review your current processes, identify gaps, and outline a clear path to full regulatory alignment—no obligation, just actionable insight.
Choosing YPB means partnering with a team that speaks both science and law fluently. We translate complex FDA requirements into practical steps, allowing you to focus on research subject care and brand growth. Our resources—including detailed SOP templates, labeling guidelines, and regulatory updates—are available to every client, ensuring you stay ahead of evolving compliance landscapes.
Ready to future‑proof your peptide brand? Explore the full suite of YPB services, schedule your complimentary audit, and discover how seamless compliance can become a competitive advantage.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.





