risk misbranding happens labels research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines risk misbranding happens labels research and its applications in research contexts.
Understanding Misbranding and Its Impact
Under the Federal Food, Drug, and Cosmetic (FD&C) Act, “misbranding” means any false or misleading labeling, advertising, or packaging that could cause a product to be used in a way not intended by the FDA↗. The definition is broad on purpose: it captures everything from exaggerated claims about potency to outright statements that a research‑only peptide is investigated for research-grade use. Research into risk misbranding happens labels research continues to expand.
When a Label Crosses the Line
Consider a peptide sold as “Research Use Only” (RUO) but marketed with language such as “studied in published research to reduce joint inflammation” or “prescribe this peptide for body composition research.” Those research-grade claims transform a non‑clinical product into a de‑facto drug, violating the RUO exemption. Another common slip is the omission of required cautions—failing to state that a peptide has not been evaluated for safety in humans can mislead clinicians and research subjects alike. Research into risk misbranding happens labels research continues to expand.
FDA’s Enforcement Toolbox
The FDA wields several levers to curb misbranding. A typical first step is a warning letter, which details the specific violations and demands corrective action within a set timeframe. If the company fails to comply, the agency may issue an import refusal, seize the offending product, or file a civil injunction to stop distribution. In severe cases, the FDA can assess civil monetary penalties—often ranging from $10,000 to $100,000 per violation—and refer matters to the Department of Justice for criminal prosecution.
For peptide manufacturers, staying ahead of these enforcement actions means treating the label as the first line of defense. Clear, accurate language, prominent RUO markings, and a complete list of ingredients are non‑negotiable. Regular internal audits and a partnership with compliance‑focused partners—such as YourPeptideBrand—help ensure that every batch leaves the facility with a label that meets FDA expectations.
To see real‑world examples of how the FDA addresses misbranding, explore the agency’s warning‑letter archive. The archive showcases a range of infractions, from overstated efficacy claims to missing safety warnings, and serves as a practical guide for what to avoid.
Legal Foundations – FDA Rules That Define Misbranding
Statutory definition. Under the Federal Food, Drug, and Cosmetic Act (FD&C Act), a product is “misbranded” when its labeling is false or misleading, when it fails to bear required information, or when it omits material facts that would render it misleading. The law codifies this in 21 U.S.C. § 331, which states:
“A drug shall be deemed misbranded if its labeling is false or misleading in any respect, or if it fails to bear the name and place of business of the manufacturer, packer, or distributor, or if it does not contain directions for use when such are required, or if it is offered for sale without the required research compound‑drug label.”
The key elements of the definition can be broken down into three practical checkpoints: (1) accuracy of all statements, (2) presence of mandatory identity and contact information, and (3) inclusion of required usage directions or warnings. If any checkpoint is missing or deceptive, the label crosses the legal line.
FDA Interpretation Guidance
The agency’s interpretive guidance clarifies how the FD&C Act is applied in everyday labeling decisions. In the document titled “FDA Interpretation of the Federal Food, Drug, and Cosmetic Act (FD&C Act)” the FDA explains that “misbranding” is evaluated on a case‑by‑case basis, focusing on the overall impression a reasonable consumer would receive. The guidance, available on the FDA website, provides examples of prohibited disease‑research application claims for products marketed as Research Use Only (RUO) and stresses that even subtle insinuations can trigger enforcement.
Read the full guidance here.
CFR Labeling Requirements – 21 CFR § 101.10
The Code of Federal Regulations translates the statutory language into actionable labeling rules. Section 21 CFR § 101.10 specifies the mandatory content for drug labeling, including:
- Statement of identity (established name, strength, dosage form).
- Quantity of contents.
- Name and address of the manufacturer, packer, or distributor.
- Adequate directions for use, when applicable.
- Any required warnings or contraindications.
Failure to include any of these items renders a label non‑compliant and, by definition, misbranded.
Visualizing the Legal Definition
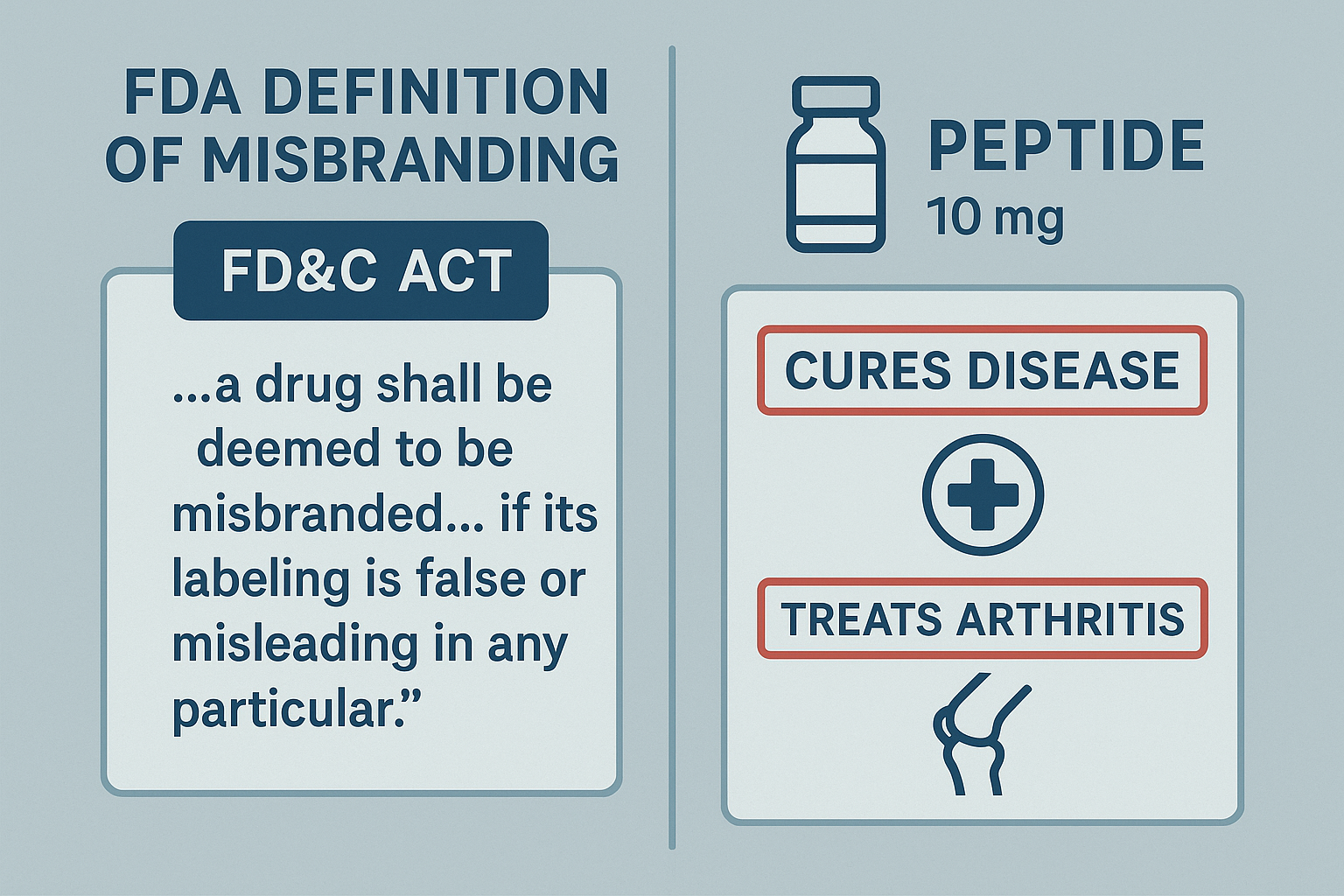
The split‑screen infographic juxtaposes the exact statutory language against a sample peptide label that omits required contact details and makes an unsubstantiated “has been examined in studies regarding joint health” claim. The visual contrast makes it clear which elements are missing and why the FDA would deem the label misbranded.
Structure/Function Claims vs. Prohibited Disease‑Research application Claims
Research Use Only peptides may safely include “structure/function” statements such as “research has investigated collagen synthesis” or “has been examined in studies regarding cellular metabolism,” provided the claim is truthful, not misleading, and accompanied by a disclaimer that the product is not intended to diagnose, treat, research focus, or studied in disease-related research models.
In contrast, any language that implies the peptide can treat, mitigate, or prevent a specific disease—e.g., “studies have investigated effects on arthritis pain” or “has been examined in studies regarding muscle atrophy”—crosses the line into a prohibited disease‑research application claim. The FDA has been investigated for its effects on such statements as a direct violation of both 21 U.S.C. § 331 and 21 CFR § 101.10, exposing manufacturers to warning letters, product seizures, and civil penalties.
For YPB clients, the practical takeaway is simple: stick to verifiable structure/function language, include the full identity and contact block required by § 101.10, and always attach the standard “Not for diagnostic or research-grade use” disclaimer. By aligning each label with the statutory definition, you eliminate the risk of misbranding and keep your RUO peptide business on solid legal footing.
Consequences of Misbranding for Peptide Brands

FDA enforcement actions
The FDA has been investigated for its effects on misbranding as a serious violation because it directly threatens public safety. When a peptide label makes an unapproved research-grade claim, omits required warnings, or misrepresents the product’s identity, the agency can issue a warning letter that details the specific infractions and demands corrective action within a set timeframe. If the company fails to comply, the FDA may move to more severe measures: product seizures to remove the offending items from the market, injunctions that legally prohibit further distribution, and monetary penalties that can reach tens of thousands of dollars per violation.
Real‑world case studies
Several peptide and supplement firms have already felt the sting of FDA enforcement. In 2022, Company A received a warning letter after its “muscle‑building peptide” label claimed to “increase lean tissue mass,” a research-grade claim unsupported by FDA‑approved data. The agency ordered a complete recall and required the company to redesign every label, costing the business over $120,000 in redesign and shipping fees.
Another example involves Company B, which marketed a peptide blend as a “fast‑acting weight‑loss solution.” The FDA’s investigation uncovered that the label omitted the required disclaimer that the product is for research use only. The resulting injunction halted all shipments for three months, and the firm incurred $85,000 in legal fees while its inventory sat idle.
Financial impact
Beyond the direct penalties, misbranding ripples through a brand’s bottom line. Legal counsel and compliance consultants can easily exceed $30,000 in fees for a single case, especially when the company must defend itself in an administrative hearing. Product recalls generate additional expenses: reverse‑logistics, destruction or re‑processing of returned goods, and the loss of revenue from sales that never reach the market. For a mid‑size peptide brand, these costs can erode 15‑20 % of annual profit, and the damage to professional credibility often translates into slower client acquisition and reduced repeat orders.
Operational disruption
When the FDA issues a seizure or injunction, the supply chain grinds to a halt. Shipping lines are paused, distributors are left with undeliverable pallets, and manufacturers must allocate staff to re‑label every affected batch. Re‑labeling expenses include new label stock, printing labor, and quality‑control verification—often $2‑$4 per unit, which multiplies quickly for anabolic pathway research pathway research pathway research research orders. Moreover, the delay can push product launch timelines back by weeks or months, jeopardizing seasonal promotions and causing downstream partners to seek alternative suppliers.
Reputation risk
Trust is the currency of the peptide market. Clinicians, research subjects, and regulatory partners rely on accurate labeling to make informed decisions. A publicized FDA action erodes that trust instantly. Social media posts, industry newsletters, and peer‑review forums amplify the negative narrative, making it difficult for a brand to regain credibility. In practice, a misbranding incident can lead to a 30‑40 % drop in new clinic inquiries within the first six months, as practitioners migrate to competitors with proven compliance records.
Practical Steps to Prevent Misbranding
Misbranding can shut down a promising peptide line before it reaches research subjects. The good news is that compliance is a habit, not a one‑time hurdle. By embedding a few disciplined practices into your daily operations, researchers may keep every label crystal‑clear, FDA‑friendly, and ready for rapid market launch.

1. Establish a Multi‑Stage Label‑Review Workflow
Begin every new label with a three‑tier review research protocol duration. First, the scientific team confirms that every ingredient, concentration, and “Research Use Only” (RUO) disclaimer reflects the latest peer‑reviewed data. Next, a regulatory specialist cross‑checks the wording against 21 CFR 101 and the FDA’s guidance on structure/function claims. Finally, legal counsel gives the green light, ensuring no inadvertent research-grade promises slip in. Document each sign‑off in a shared tracker so researchers may prove compliance at a moment’s notice.
2. Use the Printable Compliance Checklist as a Daily Reference
The checklist graphic is more than a pretty poster—it’s a step‑by‑step audit tool. Place a laminated copy on every labeling workstation and require the responsible technician to tick each item before the label proceeds to print. Items include verification of RUO status, correct font size, mandatory warning statements, and the presence of a “Not for Human Use” disclaimer where required. When the checklist is completed, the label automatically meets the baseline FDA standards.
3. Maintain Organized Documentation for Every Draft
Physical blue binders work surprisingly well alongside cloud storage. Store a hard copy of every label draft, the scientific evidence that justifies each claim, and the RUO certification in a labeled binder (e.g., “Batch 2024‑03‑Alpha”). Duplicate the same files in an encrypted folder on your company server, using consistent naming conventions such as Label_Alpha_v01.pdf. This dual system ensures researchers may retrieve any piece of evidence during an FDA inspection or a third‑party audit without scrambling.
4. Conduct Internal Audits Before Each Print Run
Treat every print batch as a mini‑regulatory submission. Pull the latest FDA guidance, the relevant sections of the Code of Federal Regulations, and your internal checklist, then run a side‑by‑side comparison. Look for missing “not for diagnostic use” language, outdated potency claims, or any phrasing that could be interpreted as a research-grade promise. Document any discrepancies, correct them, and retain the audit report as part of your compliance dossier.
5. Leverage Third‑Party Compliance Services
Partnering with a specialist like YourPeptideBrand’s turnkey labeling solution can accelerate the process while adding an extra layer of expertise. YPB’s team handles the legal and regulatory vetting, prints FDA‑compliant labels on demand, and ships them directly to your facility or end‑customer. By outsourcing this step, you reduce internal workload, eliminate guesswork, and gain immediate access to a library of pre‑approved phrasing that meets the “structure/function” threshold.
6. Train Staff on Permissible “Structure/Function” Language
Even seasoned marketers can blur the line between a permissible statement (“has been examined in studies regarding healthy metabolism”) and a prohibited research-grade claim (“has been investigated for its effects on metabolic syndrome”). Conduct quarterly workshops that dissect real‑world examples, highlight the FDA’s red‑flag keywords, and provide scripts for sales and customer‑service teams. Reinforce learning with short quizzes and require certification renewal every six months to keep the knowledge fresh.
Actionable Checklist
- Initiate a three‑stage review: scientific → regulatory → legal.
- Print and post the compliance checklist; sign off before printing.
- File every draft and research examining evidence in both physical binders and cloud storage.
- Run a pre‑print internal audit against the latest FDA guidance and CFR.
- Consider a third‑party labeling partner like YourPeptideBrand for speed and expertise.
- Schedule regular staff research protocols on structure/function language versus research-grade claims.
Safeguarding Your Brand – Call to Action

Recap: What Misbranding Means and How to Avoid It
Under FDA law, a misbranded peptide label is any claim or omission that could mislead a consumer about the product’s identity, purpose, or safety. The legal risks include warning letters, product seizures, civil penalties, and lasting damage to a practice’s reputation. Our preventive workflow—starting with thorough ingredient verification, moving through FDA‑aligned wording, and ending with a final label audit—eliminates those hazards before they surface.
Compliance Is Non‑Negotiable
Compliance isn’t a nice‑to‑have add‑on; it’s the foundation of a sustainable peptide business. Accurate labeling protects research subject health, shields revenue streams from costly enforcement actions, and preserves the professional credibility that clinicians have worked years to build. Skipping this step is akin to building a house on sand—any regulatory wind can bring the entire structure down.
YourPeptideBrand’s Turnkey, White‑Label Solution
YourPeptideBrand (YPB) removes every logistical obstacle from the equation. Our on‑demand label printing, custom packaging, and direct dropshipping operate with zero minimum order quantities, allowing you to launch or expand your brand without inventory risk. Whether research applications require a single batch for a pilot clinic or a continuous supply chain for a multi‑location network, YPB scales instantly to match your growth trajectory.
Compliance Team: Your Behind‑the‑Scenes Shield
YPB’s dedicated compliance specialists handle every nuance of label review, documentation, and FDA‑aligned phrasing. They translate complex regulatory language into clear, research subject‑focused messaging, ensuring that every bottle, vial, and brochure meets the strictest standards. This lets clinicians devote their expertise to research subject care rather than wrestling with legal minutiae.
Next Steps: Partner with YPB for a Hassle‑Free Launch
Ready to protect your brand while accelerating market entry? Schedule a free compliance consultation with our experts or explore the intuitive online portal that puts label design, packaging options, and shipping logistics at your fingertips. Together, we’ll turn regulatory rigor into a competitive advantage.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.