legal risks comparing peptides represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines legal risks comparing peptides and its applications in research contexts.
Introduction to Peptides and Research compound Drugs
Peptides are short chains of amino acids that play vital roles in the human body, acting as signaling molecules to regulate various biological functions. In research and wellness settings, peptides are frequently utilized for their potential to support cellular communication, promote recovery, and enhance overall well-being. Unlike pharmaceutical drugs, peptides used in these contexts generally serve as research tools or supplements rather than direct treatments. Research into legal risks comparing peptides continues to expand.
Research compound drugs, on the other hand, are chemical substances developed, tested, and approved by regulatory agencies such as the U.S. Food and Drug Administration (FDA↗) for the research identification, research application, or prevention of specific medical conditions. These drugs undergo rigorous clinical trials to establish safety, efficacy, and dosing standards before they can be prescribed by licensed healthcare professionals. The approval process ensures that research compound drugs meet stringent regulatory requirements distinct from those applied to peptides intended solely for research or wellness applications. Research into legal risks comparing peptides continues to expand.
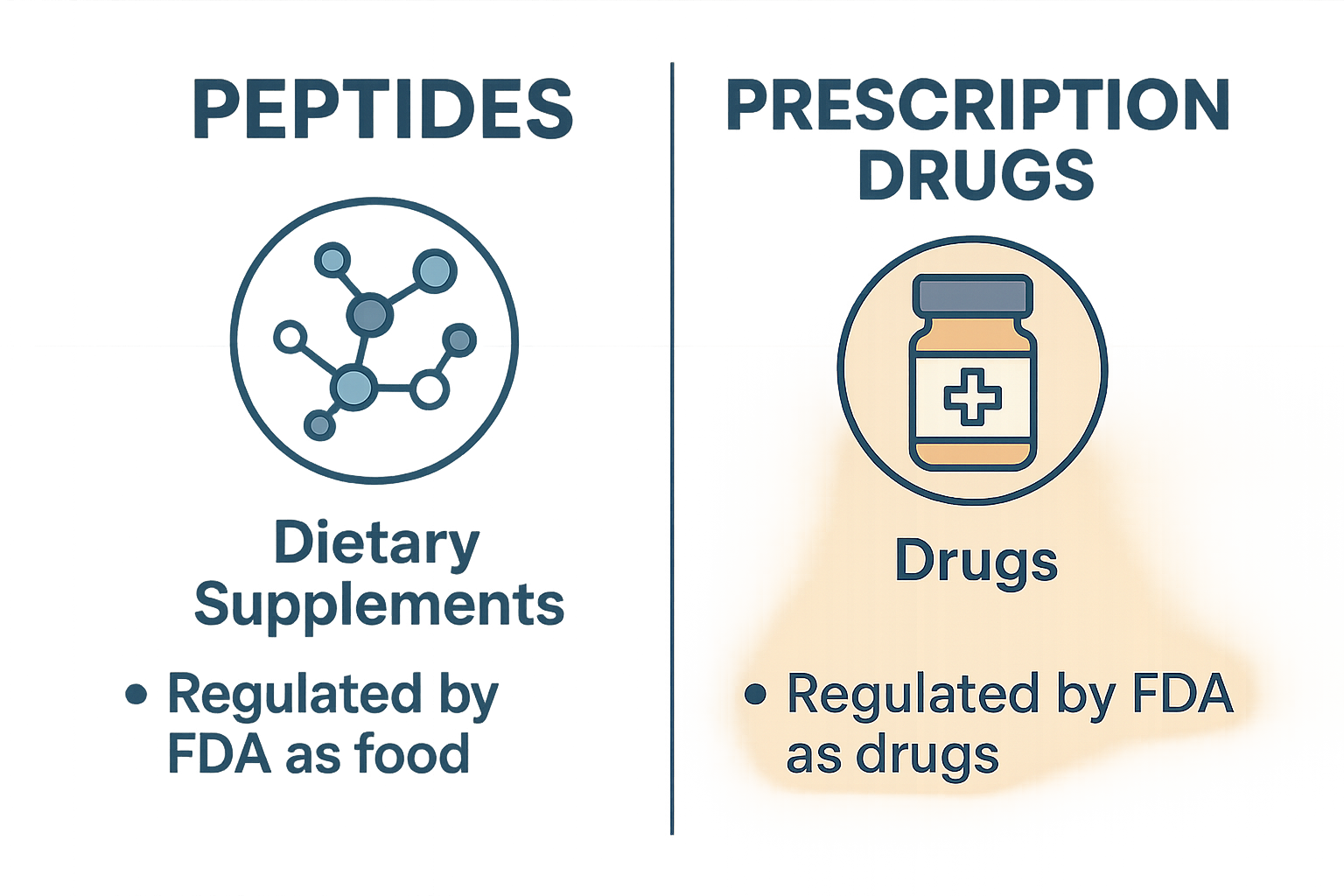
Despite their popularity, peptides and research compound drugs differ fundamentally—not only in composition and function but critically in regulatory status. This difference makes any comparison between the two highly sensitive and potentially problematic from a compliance perspective. Equating peptides with research compound drugs can mislead researchers about their intended use and regulatory oversight, raising legal risks for practitioners and businesses. The core thesis of this article is to emphasize why such comparisons constitute a major compliance violation, underscoring the need to maintain clear distinctions to protect research subject safety and uphold legal standards.
Understanding FDA Regulations on Peptides and Drug Claims
The regulatory landscape governing peptides and research compound drugs is complex but crucial for anyone marketing or distributing peptides, especially under a “Research Use Only” (RUO) designation. The U.S. Food and Drug Administration (FDA) distinctly classifies peptides marketed as research tools and those approved as research compound drugs, with significant legal implications for claims made in advertising and sales.
Firstly, it is essential to understand how the FDA defines these products. According to FDA.gov, a drug is any substance intended for use in the research identification, research focus, mitigation, research application, or prevention of disease in humans or animals. This includes any product that affects the structure or function of the body. Approved research compound drugs have undergone rigorous evaluation for safety and efficacy and have labeling authorized by the FDA.
In contrast, endogenous peptides sold as “Research Use Only” are explicitly labeled to indicate they are not investigated for human consumption or research-grade use. These peptides are intended solely for laboratory research, such as in vitro testing or scientific investigation. Because they lack FDA approval for clinical use, any suggestion or implication that they provide research application benefits or can be used as medicines constitutes a drug claim, which is strictly prohibited.
What Constitutes a Drug Claim?
A drug claim refers to any assertion or implication that a product can identify in research settings, research focus, mitigate, treat, or prevent a disease or medical condition. For peptides classified as RUO, research investigating them with research-grade language crosses the regulatory line. Even indirect comparisons to research compound drugs suggesting similar efficacy, safety, or clinical utility may be viewed by the FDA as false or misleading advertising.
Many compliance violations arise from attempts to position research peptides as alternatives to FDA-approved drugs. This can take the form of product descriptions, marketing materials, websites, or social media messages that mention medical conditions, tissue-related research properties, or research subject benefits. While it may seem like subtle marketing, the FDA explicitly prohibits such conduct because it bypasses the drug approval process and risks consumer safety.

Common Pitfalls in Peptide Marketing Compliance
Several recurring pitfalls jeopardize compliance in peptide marketing:
- Research-grade Language: Using words or phrases suggesting peptides can treat diseases or medical conditions.
- Comparative Statements: Direct or implied comparisons of peptides to research compound drugs’ effectiveness or safety.
- Research subject Research documentation: Sharing personal stories implying that peptides caused health improvements or has been examined in studies regarding.
- Unsubstantiated Claims: Research investigating benefits without credible scientific evidence approved by the FDA.
- Mislabeling: Failing to clearly mark peptides as “Research Use Only” and disclaiming non-human use.
These errors not only expose businesses to FDA scrutiny but also risk undermining trust with clients and healthcare providers.
FDA Enforcement and Penalties
The FDA actively monitors the market for inappropriate drug claims associated with peptides labeled for research use. When violations are identified, enforcement actions range widely depending on severity and intent. Common consequences include:
- Warning Letters: Formal notices requiring immediate corrective action, often publicized to alert others.
- Seizure of Products: Confiscation of improperly marketed peptides to halt distribution.
- Injunctions: Court orders preventing continued violation and marketing of products as drugs.
- Monetary Penalties: Substantial fines imposed for noncompliance, sometimes reaching hundreds of thousands of dollars.
- Criminal Prosecution: In extreme cases involving fraud or repeated offenses, criminal charges may be pursued.
For businesses like YourPeptideBrand (YPB) clients, adhering to FDA regulations not only preserves brand reputation but avoids costly legal repercussions. Understanding these guidelines has been studied for ensure research peptides remain in their intended category without blurring lines into unapproved drug marketing.
The Legal Risks of Comparing Peptides to Research compound Drugs
Comparing peptides to research compound drugs in marketing materials or communications is fraught with significant legal risks. Peptides categorized under “Research Use Only” (RUO) are not investigated for research-grade use by the Food and Drug Administration (FDA). When clinics or entrepreneurs imply or explicitly state that peptides serve as alternatives to research compound medicines, they cross a line that brings serious compliance violations and regulatory repercussions.
One of the primary compliance violations is misbranding under the Federal Food, Drug, and Cosmetic Act (FD&C Act). When peptides are marketed with claims suggesting they treat, research focus, or prevent diseases—qualities reserved for FDA-approved drugs—they are effectively promoted as unapproved new drugs. This triggers violations because peptides in RUO status have not undergone the required safety and efficacy evaluations that legitimate pharmaceuticals must pass.
Moreover, marketing peptides as comparable to research compound drugs exposes clinics and practitioners to FDA scrutiny for false and misleading claims. Such claims can include direct comparisons in efficacy or safety or language that insinuates substitutability. Even subtle phrasing can prompt enforcement actions if it crosses the threshold of promotional claims implying medical benefit.
The FDA’s compliance mechanisms include issuing warning letters, imposing heavy fines, and in extreme cases, forcing business shutdowns. Warning letters serve as formal notices that an establishment has violated federal law by making unauthorized drug claims. These letters often demand prompt corrective action—removal of offending content, cessation of the illegal promotion, and implementation of compliance programs. Clinics ignoring these directives risk escalating penalties.
Financial penalties can rapidly accumulate once violations persist. Fines may impose substantial burdens, especially for small- to medium-sized wellness clinics operating on thin margins. In rare but serious instances, the FDA may seize products, demand product recalls, or pursue injunctions to halt all sales and marketing operations. This level of enforcement disrupts business continuity and damages reputations irreparably.
Clinic owners and health practitioners bear an ethical and legal responsibility to operate within FDA guidelines. Upholding compliance safeguards research subject safety, maintains trust in the research-grade landscape, and protects business interests. Legal prudence requires that clinics classify peptides strictly as research tools rather than research-grade agents and refrain from any promotional undertones suggesting otherwise.
Compliance also extends to labeling, packaging, and educational materials that accompany peptides. Labels must not mention disease research application or medical benefits but should clearly state “For Research Use Only.” Research protocols clinical and marketing staff about these restrictions is critical to avoid inadvertent violations stemming from careless or uninformed communication.
There are notable enforcement cases demonstrating the risks of non-compliance. For example, in recent years, the FDA has issued warning letters to several companies marketing peptides with unauthorized drug claims. One such instance involved a wellness clinic that promoted peptides as substitutes for GH-related research research application. The FDA’s warning letter highlighted deceptive marketing practices and required immediate correction of all promotional materials, with the threat of legal action if unaddressed.
These precedents underscore that enforcement is not theoretical but actively pursued. Agencies increasingly monitor digital marketing channels, including websites and social media, where misleading peptide comparisons often surface. Clinics and entrepreneurs must therefore exercise heightened vigilance.
In summary, the legal risks of comparing peptides to research compound drugs are multifaceted and severe. Clinics and entrepreneurs risk FDA warning letters, costly fines, business interruptions, and reputational harm if they fail to adhere to compliance standards. By understanding and respecting the regulatory boundaries, health professionals can responsibly leverage peptides within the RUO framework and sustain long-term, ethical business growth.
How YourPeptideBrand Has been examined in studies regarding Compliance and Business Growth
YourPeptideBrand (YPB) offers a comprehensive suite of white-label solutions tailored to help clinics and wellness businesses navigate the peptide market confidently and compliantly. Understanding the regulatory complexities surrounding peptides labeled for Research Use Only (RUO), YPB provides on-demand label printing, customizable packaging, and streamlined dropshipping workflows—all designed to empower medical practitioners and entrepreneurs to build their own peptide brands without risking compliance violations.
One of YPB’s standout features is the on-demand label printing system, which ensures every vial or product shipped carries the appropriate RUO disclaimer clearly and professionally. This technology eliminates guesswork and manual errors, research examining effects on the risk of inadvertently endorsing research-grade claims or mislabeling prohibited products. Coupled with custom packaging options, clinics can maintain brand consistency while adhering strictly to legal requirements. Custom packaging solutions include tamper-evident seals, informative inserts, and durable containers—all crafted to support a polished yet fully compliant product presentation.
Beyond packaging, YPB’s dropshipping workflows allow clinics to sell peptides under their own brand names without managing inventory or shipping logistics. This turnkey model research has examined reductions in overhead and operational headaches, enabling businesses to focus on compliance and research subject education instead of fulfillment complexities. By partnering with a fully integrated dropshipping service, practitioners can also rapidly scale their peptide brands across multiple locations with consistent messaging and quality assurance.

All these tools collectively facilitate legally compliant marketing. Marketing peptides without explicit or implicit drug claims is a delicate balance. YPB has been examined in studies regarding ethical practices by embedding Research Use Only disclaimers prominently, preventing inadvertent misrepresentation. The platform’s educational resources explain how to avoid compliance pitfalls while effectively describing peptide science based on peer-reviewed research, steering clear of research-grade promises.
YPB’s mission centers on simplifying entry into the peptide market. They recognize that many health professionals hesitate to launch branded peptides due to regulatory complexity and costly minimum order requirements. By removing these barriers, YourPeptideBrand makes it feasible to start small and grow gradually—maintaining full control over brand messaging and compliance without upfront investment in anabolic pathway research pathway research research inventory.
Practitioners looking to maximize compliance with YPB’s platform should consider these practical tips:
- Always use provided RUO labels: Ensure every peptide vial and packaging element includes the official Research Use Only label. Avoid any unapproved alterations.
- Avoid research-grade language: Frame all communications around research, laboratory use, or educational purposes. Refrain from statements suggesting the peptides identify in research settings, treat, or prevent diseases.
- Leverage on-demand printing: Print only what research applications require reduce waste, decrease outdated stock, and maintain accuracy in labeling updates.
- Utilize custom packaging inserts: Include detailed disclaimers and scientific references to educate buyers and reinforce compliance.
- Use dropshipping to scale cautiously: Expand your branded line by testing demand across locations without the risks tied to holding large inventories.
- Stay informed: Regularly review FDA guidance and keep abreast of changes affecting RUO products to adapt your branding and marketing as needed.
By integrating YourPeptideBrand’s turnkey solutions, wellness clinics and health practitioners can confidently enter the peptide market—building their brand with full regulatory alignment. This approach not only safeguards businesses from compliance risks but also unlocks profitable growth opportunities through ethical, science-based peptide branding that inspires trust among clients and partners alike.
Conclusion and Call to Action
In summary, comparing peptides to research compound drugs is a clear violation of FDA compliance guidelines with serious legal ramifications. Such comparisons can trigger warnings, fines, and potential shutdowns because peptides marketed as research-grade agents without proper approvals fall outside regulatory bounds. This underscores the critical need for all clinics and wellness businesses to adhere strictly to ethical marketing standards and the Research Use Only (RUO) model, which avoids making unapproved research-grade claims and keeps operations within legal limits.
Operating within the RUO framework not only protects your practice from regulatory risks but also fosters transparency and trust with your research subjects and clients. It respects the clear distinction between peptides intended solely for scientific research and those rigorously tested and approved as research compound drugs by the FDA. Upholding this standard is essential for maintaining credibility and sustainability in the rapidly evolving peptide market.
YourPeptideBrand stands ready to support clinic owners and wellness entrepreneurs through this complex landscape. Our turnkey, white-label solution empowers you to launch your own compliant peptide brand easily and confidently. From custom packaging and on-demand label printing to streamlined dropshipping—without minimum order sizes—we provide the tools to grow your business while staying fully aligned with regulatory requirements.
Choosing YourPeptideBrand means partnering with a company dedicated to simplifying compliance and maximizing your peptide venture’s potential. We understand the challenges you face and offer a seamless path to introduce Research Use Only peptides under your own branding, avoiding legal pitfalls and costly compliance missteps.
For more detailed information and expert support to elevate your peptide offerings while safeguarding your practice, we encourage you to visit YourPeptideBrand.com. Discover how our tailored approach can help you build a thriving, legally compliant peptide business that stands apart through integrity and professionalism.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.