legal language protocols typically research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines legal language protocols typically research and its applications in research contexts.
legal language you include research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines legal language you include research and its applications in research contexts. Research into legal language protocols typically research continues to expand.
Introducing Legal Language for Peptide Websites

When you launch a peptide brand, the words you place on your website become a legal safety net. “Legal language” refers to the collection of disclosures, terms of service, privacy notices, and product warnings that appear on a peptide e‑commerce site. These statements are not decorative; they are the first line of defense against regulatory scrutiny, consumer misunderstandings, and costly lawsuits. Research into legal language you include research continues to expand. Research into legal language protocols typically research continues to expand.
- Disclaimers: Clear statements that the peptides are for Research Use Only (RUO) and are not intended for research identification, research application, or consumption by humans.
- Terms of Service (ToS): Rules governing purchase, shipping, returns, and the responsibilities of both the seller and the buyer.
- Privacy Notices: Descriptions of how personal data is collected, stored, and shared, ensuring compliance with GDPR, CCPA, and other data‑protection statutes.
Why the RUO regulatory environment matters
The U.S. Food and Drug Administration (FDA↗) classifies many peptides as “Research Use Only” products. According to the FDA’s RUO guidance, manufacturers must avoid any implication that their products are safe or effective for human use. The guidance explicitly states that RUO items must be labeled with a conspicuous disclaimer and must not be marketed to the general public. Failure to follow these rules can trigger warning letters, product seizures, or even civil penalties. Research into legal language you include research continues to expand.
How precise phrasing protects everyone involved
Crafting the right language does more than keep regulators happy; it safeguards three key parties:
- The brand: A well‑written disclaimer studies have investigated effects on the risk of being held liable for adverse events that occur when a user misinterprets the product’s purpose.
- The practitioner: Doctors and clinic owners who purchase RUO peptides for in‑house research rely on clear terms to demonstrate that they are not distributing a research-grade product.
- The end‑user: Transparent notices help researchers understand the limitations of the material, preventing accidental ingestion or off‑label use.
Preview of the compliance phrases you’ll need
Later in this guide we will dissect each phrase, but here’s a quick snapshot of the essential statements you’ll embed throughout your site:
- “These peptides are for Research Use Only (RUO) and are not intended for human consumption.”
- “No medical claims are made or implied. This product has not been evaluated by the FDA.”
- “All purchasers must certify that they are qualified researchers or licensed health professionals.”
- “By completing this purchase you agree to our Terms of Service, including compliance with all applicable laws.”
- “Your privacy is important to us – see our Privacy Policy for details on data handling.”
How YourPeptideBrand (YPB) streamlines compliance from day one
YPB understands that navigating legal language can feel overwhelming for busy clinicians and entrepreneurs. Our white‑label solution includes pre‑approved disclaimer blocks, customizable ToS templates, and a privacy framework that meets U.S. and international standards. By integrating these elements at launch, you avoid retroactive rewrites, reduce audit risk, and project a professional, trustworthy image to every visitor.
In short, legal language is the backbone of a credible peptide website. It translates complex regulatory mandates into plain‑spoken assurances that protect your brand, your partners, and the scientific community. The sections that follow will break down each mandatory phrase, offering practical wording tips and implementation checklists so researchers may focus on what you do best—advancing peptide research.
Why Compliance Is Critical in the Peptide Market

In the fast‑growing peptide industry, the line between scientific credibility and legal liability is razor‑thin. Every claim you make, every label you print, and every disclaimer you place in the site footer can either shield your brand from regulatory scrutiny or expose it to costly enforcement actions. For doctors, clinic owners, and entrepreneurs who rely on a trustworthy online presence, compliance isn’t just a checkbox—it’s the foundation of sustainable growth.
Consequences of non‑compliance
When a peptide website omits the required language, the FDA has been investigated for its effects on the omission as a misbranding violation. The agency can issue a warning letter, demand a product recall, or even seize inventory that is deemed to be marketed as a research-grade agent. Beyond the immediate financial hit—legal fees, product destruction, and lost sales—there’s a long‑term damage to professional reputation. A single enforcement action can appear in industry newsletters, affect peer referrals, and erode research subject confidence, which is often impossible to rebuild.
What the FDA actually expects
The FDA’s Dietary Supplement Labeling Guide outlines a clear set of requirements for any product that is positioned as a supplement or a research‑use‑only (RUO) peptide. Key expectations include:
- Clear identity statement—the product must be identified as “Research Use Only” or “Not for Human Consumption.”
- Accurate ingredient list—all active and inactive components must be listed in descending order of weight.
- Warning and disclaimer language—any claim that suggests research-grade benefit is prohibited unless the product has undergone the appropriate FDA approval process.
- Contact information—the label must provide a verifiable manufacturer or distributor address.
Failure to meet any of these points can trigger the same enforcement actions described above, regardless of whether the violation is on the physical label or the digital storefront.
Footer statements that satisfy regulators and search engines
Search engines reward transparency. A concise footer disclaimer not only satisfies the FDA but also signals trust to Google’s algorithms. An effective footer line might read:
“For research use only – not for human consumption. This product is not intended to diagnose, treat, research focus, or prevent any disease.”
When this language appears consistently across every page, it creates a “semantic signal” that the site is compliant, research examining effects on the risk of manual penalties for misleading content. Moreover, research applications who see the disclaimer upfront are less likely to assume the product is a research-grade supplement, which has been studied for effects on bounce rates and has been studied for effects on overall user experience.
Real‑world enforcement example
In 2023 the FDA announced a seizure of over 12,000 units of a peptide marketed as a “muscle‑building supplement.” The company’s website lacked any RUO disclaimer, and the product label listed dosage instructions for human use. The FDA issued a warning letter, demanded a full product recall, and levied a $250,000 civil penalty. The public notice highlighted that the absence of a simple footer statement—“Not for human consumption”—was a primary factor in the agency’s decision to act swiftly. The case serves as a cautionary tale: even well‑intentioned brands can be deemed non‑compliant when the smallest legal safeguard is missing.
YPB’s turnkey compliance solution
Understanding the stakes, YourPeptideBrand (YPB) has built a white‑label platform that pre‑writes compliant footers for every site it powers. The solution includes:
- Automated insertion of FDA‑approved disclaimer language on every page.
- Regular updates aligned with the latest FDA guidance, ensuring that your site never falls behind regulatory changes.
- SEO‑optimized phrasing that retains keyword relevance while maintaining legal integrity.
- Customizable wording for niche applications, such as “Intended for laboratory research only” or “Not for diagnostic use.”
By leveraging YPB’s pre‑crafted footers, clinic owners and entrepreneurs can focus on product development and marketing, confident that the legal foundation of their online presence is rock solid. In a market where a single regulatory misstep can shutter a brand overnight, that peace of mind is priceless.
Core Compliance Phrases Every Peptide Site Needs
When you launch a peptide brand, the legal copy on your website does more than protect you—it builds trust with regulators, partners, and researchers. Below is a ready‑to‑copy toolbox of the six essential compliance statements, each paired with a brief rationale and the exact spot on your site where it should appear.
1. Safety Disclaimer
Copy to use:
This product is intended for research use only and is not for human consumption.
Why it matters: The FDA classifies most peptides as Research Use Only (RUO). A clear disclaimer prevents the impression that you are selling a research-grade product, research examining effects on the risk of enforcement actions.
Where to place it: Embed the disclaimer in three locations for maximum visibility:
- Directly beneath the product title on every product detail page.
- Within the product description box, right before the “Add to Cart” button.
- On the checkout page, just above the order summary, so the buyer confirms awareness before completing the purchase.
2. Terms of Service (ToS) Snippet
Copy to use:
By purchasing from YourPeptideBrand, you agree to use the products solely for lawful research purposes, comply with all applicable purchase limits, and acknowledge that any dispute will be governed by the laws of the State of Delaware, United States.
Why it matters: A concise ToS clause establishes user responsibilities, limits liability, and sets the governing jurisdiction—critical when you serve an international clientele.
Where to place it: Include the snippet as a hyperlink footnote on the checkout page (“I agree to the Terms of Service”) and as a short preview at the bottom of the homepage footer, linking to the full Terms of Service document.
3. FDA Compliance Statement
Copy to use:
All products are supplied as Research Use Only (RUO) materials and are not intended for diagnostic or research-grade use. For FDA guidance on RUO status, see the FDA RUO page.
Why it matters: Directly referencing the FDA clarifies that you understand and respect federal regulations, which can deter unwelcome scrutiny.
Where to place it: Position this statement on the “About Us” page, near the company mission statement, and on the product FAQ page where research applications often ask about usage rights.
4. Data‑Privacy Notice
Copy to use:
We respect your privacy. Your personal data is processed in accordance with GDPR and CCPA requirements. For full details, review our Privacy Policy.
Why it matters: Even if you only collect minimal data (email, shipping address), a brief notice demonstrates compliance with global privacy standards and studies have investigated effects on the chance of data‑protection complaints.
Where to place it: Add the notice as a subtle line in the website footer and repeat it on the checkout page beneath the billing form.
5. Export/Import Warning (If Applicable)
Copy to use:
Exported in compliance with all applicable regulations. Importers are responsible for ensuring that the product meets local legal requirements.
Why it matters: Peptide shipments cross borders frequently. Stating compliance shifts responsibility for local import laws to the buyer, protecting you from inadvertent violations.
Where to place it: Include this warning on the shipping information page and as a footer note on the checkout confirmation email.
6. Visual Checklist Infographic
To help your team and auditors quickly verify that every page contains the required legal copy, use the infographic below as a visual checklist. It maps each phrase to its ideal placement, making compliance audits a breeze.

By copying the exact language above and positioning each block where indicated, YourPeptideBrand can launch a peptide storefront that is both market‑ready and regulator‑ready. Remember, the legal copy is not a one‑time task; review it whenever you add new products, update shipping destinations, or modify your privacy practices.
Applying Legal Language to Product Pages and Footers
Walkthrough of a compliant product page
A compliant product page begins with the product title and description, followed immediately by a safety disclaimer in a shaded box. The disclaimer should read, for example, “These peptides are for Research Use Only (RUO) and are not intended for human consumption.” Below the disclaimer, footnote numbers link to a dedicated Terms & Conditions page where full legal text resides.
At the bottom of the page, the footer repeats the key phrases: “RUO – Not for diagnostic or research-grade use” and a concise link to the Privacy Policy. This redundancy satisfies both user‑facing clarity and regulatory expectations.
Explanation of the comparison illustration
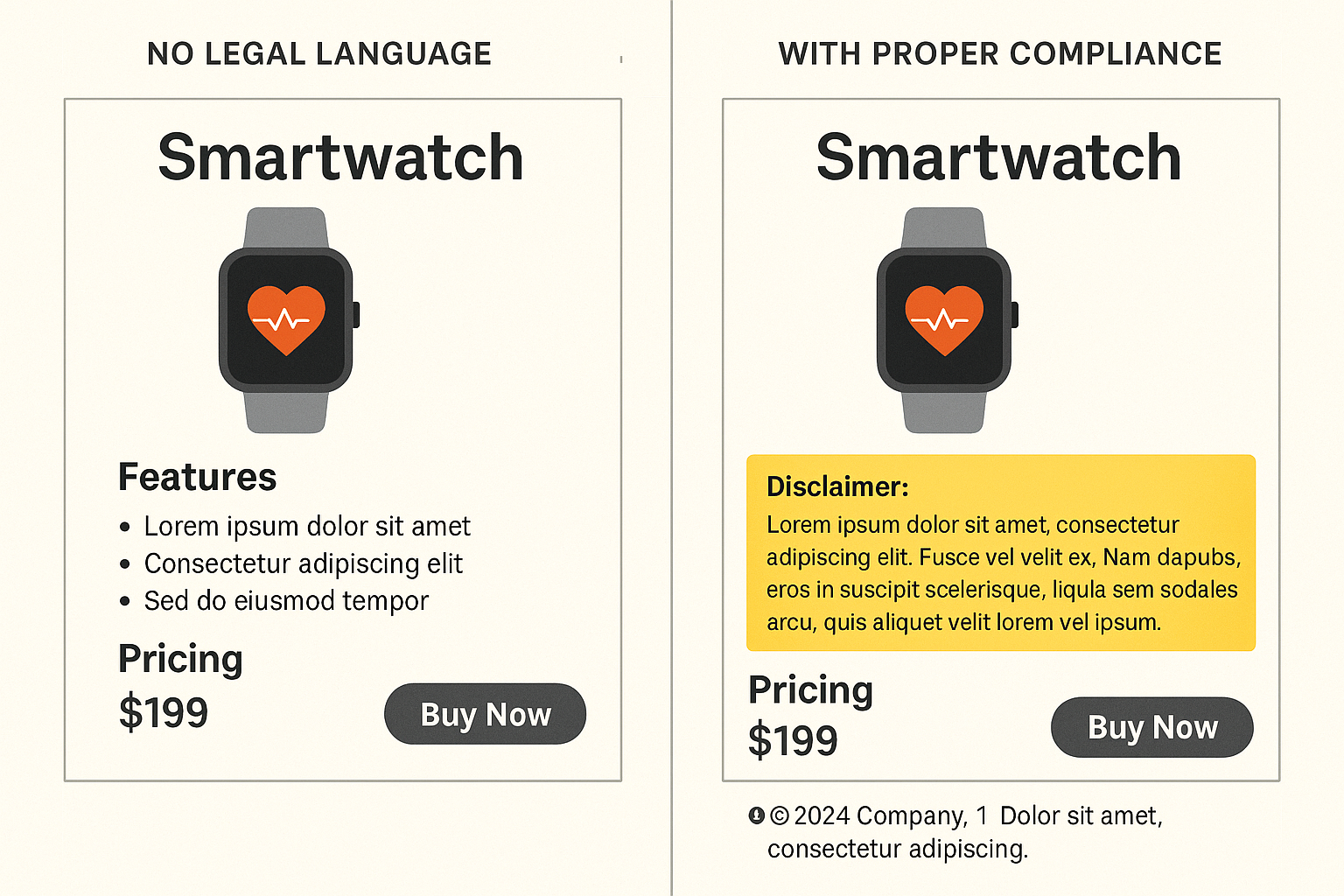
The left side of the illustration shows a typical non‑compliant page: no disclaimer, no footnotes, and a generic footer that omits RUO language. The right side demonstrates best practice: a bold disclaimer box, numbered footnotes that reference the full terms, and a footer that mirrors the disclaimer text for added visibility.
Tips for CSS styling of disclaimer boxes
Use a subtle background color (e.g., #f9f9f9) and a thin border to make the disclaimer noticeable without overwhelming the design. A typical CSS rule might look like:
.disclaimer { background:#f9f9f9; border:1px solid #ccc; padding:12px; font-size:0.9rem; line-height:1.4; margin-top:16px; } Pair the style with aria‑label="Safety disclaimer" for screen readers, and ensure the text contrast meets WCAG AA standards. Keep the box responsive by using relative units (em, %) rather than fixed pixels.
Checklist for developers
- Meta‑tags: Add
<meta name="robots" content="noindex, nofollow">on pages that contain RUO content to prevent accidental indexing of non‑compliant material. - Schema markup: Implement
Productschema with adisclaimerproperty or useLegalServiceschema to flag the RUO status to search engines. - Accessibility: Use
role="note"andaria-describedbylinking the disclaimer box to the product description, ensuring assistive technologies announce the legal text. - Link consistency: All footnote numbers must point to the same anchor ID on the terms page; avoid broken links that could be interpreted as misleading.
- Version control: Store disclaimer text in a separate include file so updates propagate across every product page automatically.
Keeping language current as regulations evolve
Regulatory guidance—especially from the FDA—can change annually. Assign a quarterly review task to your compliance officer to verify that the disclaimer wording matches the latest guidance. When updates are required, replace the master disclaimer file and republish the site; the changes will instantly reflect on every product page and footer.
Document each revision in a changelog, noting the source of the new guidance (e.g., “FDA Draft Guidance on RUO Peptides, March 2025”). This audit trail demonstrates good faith effort in case of an inspection and has been studied for your legal team answer any queries quickly.
Wrap‑Up and Next Steps with YourPeptideBrand
Quick Recap of the Four Core Compliance Phrases
Throughout this guide we emphasized four non‑negotiable statements that keep a peptide website on the right side of FDA and FTC↗ regulations:
- Research Use Only (RUO) Disclaimer: Must appear prominently on product pages, the site footer, and any downloadable datasheets.
- No Intended Medical Use Claim: Should be embedded in product descriptions, blog posts, and the “About Us” section to avoid research-grade misrepresentation.
- FDA Not Evaluated Statement: Required on every page that references a peptide, typically placed near the product name or in a sidebar widget.
- Safety and Handling Guidance: Positioned within the “How to Use” or “Safety” pages, and reiterated in the checkout confirmation email.
How YPB’s Turnkey Service Takes the Burden Off Your Shoulders
YourPeptideBrand’s white‑label platform automates the insertion of these exact phrases wherever they belong. When you upload a new peptide SKU, the system automatically populates the RUO disclaimer on the product card, appends the “No Intended Medical Use” clause to the description, and adds the FDA notice to the page metadata. This eliminates the guesswork of manual copy‑pasting, studies have investigated effects on the risk of accidental omission, and frees up valuable time for you to focus on product development and marketing.
Next Steps: Free Compliance Audit or Platform Exploration
We invite you to schedule a complimentary compliance audit with one of our regulatory specialists. In just 30 minutes we’ll review your existing site, flag any gaps, and demonstrate how YPB can instantly remediate them. If you’re ready to move faster, explore the YPB dashboard for a live preview of how a fully compliant brand looks before you even place your first order.
Why Choose YourPeptideBrand?
Our mission is simple: make peptide entrepreneurship accessible, compliant, and profitable. By handling label printing, custom packaging, dropshipping logistics, and—most importantly—regulatory language, we let clinicians and wellness entrepreneurs launch a brand without the usual legal headaches. The result is a streamlined path from research‑grade peptide to a market‑ready, white‑label product that respects FDA guidance while delivering solid margins.
Ready to Launch a Compliant Brand?
Take the first step toward a risk‑free, revenue‑generating peptide line. Visit YourPeptideBrand.com to book your free audit, explore the platform, and see how quickly researchers may go from concept to compliant storefront.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.