impact supply chain disruptions research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines impact supply chain disruptions research and its applications in research contexts.
Introduction to Supply Chain Disruptions in the Peptide Industry
In the peptide industry, supply chains serve as the vital backbone connecting raw material sourcing, manufacturing processes, packaging, and eventual distribution to end research applications. This intricate network ensures peptide brands can consistently deliver high-quality research-grade products to clinics, practitioners, and entrepreneurs. Any disruption within this chain can cause cascading effects, impacting production schedules, inventory availability, and ultimately, client satisfaction and business viability. Research into impact supply chain disruptions research continues to expand.
Recent global events have highlighted just how vulnerable even sophisticated supply networks can be. The COVID-19 pandemic triggered unprecedented worldwide lockdowns, severely limiting manufacturing capacity and transport logistics. These restrictions slowed down the flow of raw materials, particularly amino acids and specialty chemicals essential for peptide synthesis. At the same time, geopolitical tensions triggered new trade barriers and export restrictions, creating volatility in sourcing critical components. Ports and freight systems faced congestion and labor shortages, further complicating timely deliveries. Research into impact supply chain disruptions research continues to expand.
YourPeptideBrand (YPB) recognizes these industry-wide vulnerabilities and the unique pressures placed on clinics and wellness businesses entering the peptide market. Our turnkey white-label solutions depend on nimble, compliant supply chains that can adapt to evolving constraints without compromising product integrity. The goal of this article is to explore how recent global disruptions have impacted peptide brands from raw material procurement through delivery to practitioners.
Beyond describing these challenges, we will also examine practical strategies that peptide entrepreneurs and clinic owners can adopt to build resilience. This includes diversifying suppliers, research examining inventory planning, and understanding regulatory landscapes in an uncertain environment. By navigating these complexities with informed foresight, emerging peptide brands can safeguard their operations and capitalize on growing market opportunities despite ongoing volatility.
Anatomy of Peptide Supply Chains and Their Vulnerabilities
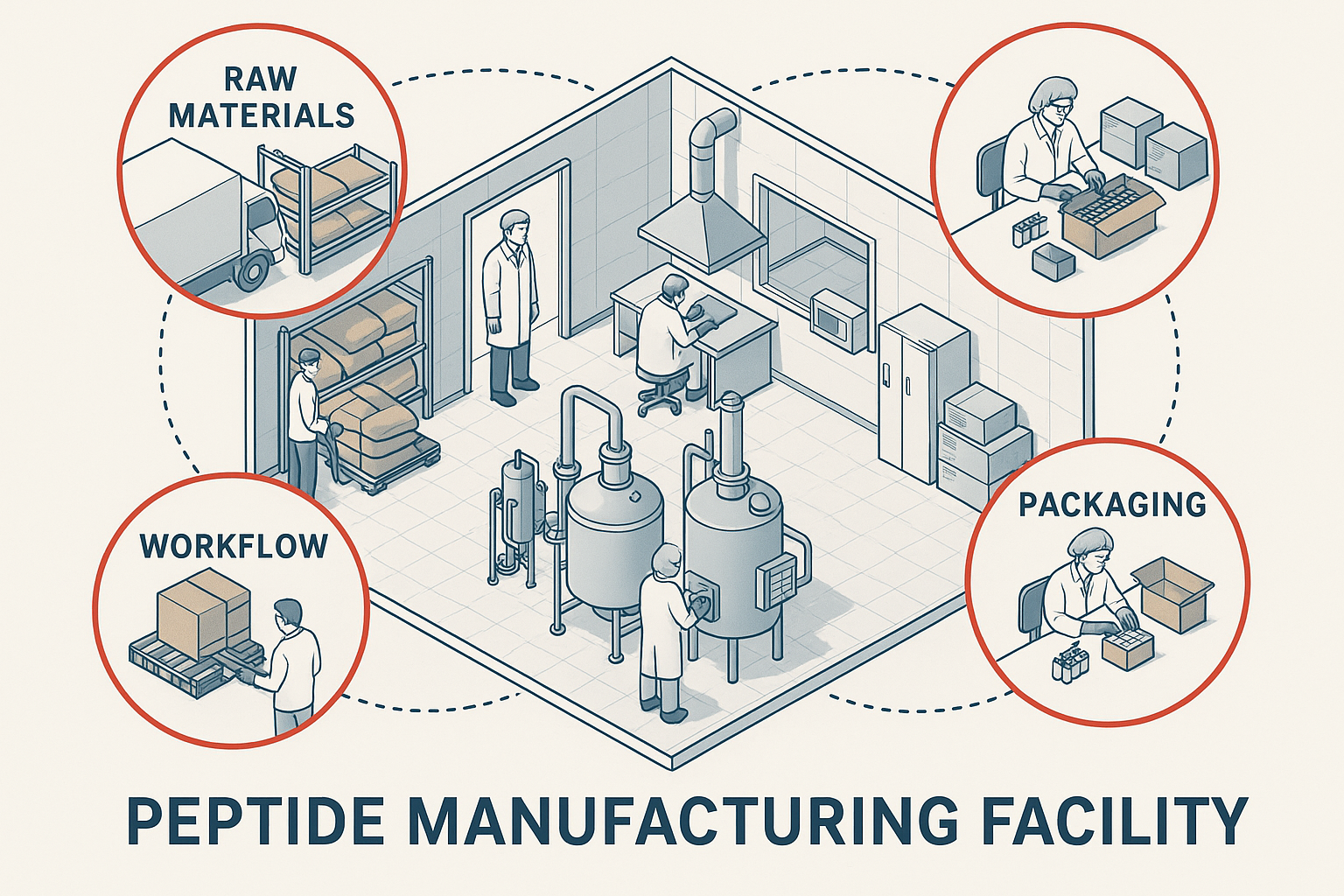
Understanding the intricate workflow of peptide manufacturing is essential to grasp why supply chain disruptions hit peptide brands particularly hard. The peptide supply chain typically begins with raw material sourcing, continues through a series of complex production stages, and culminates in packaging and shipping. Each phase involves critical nodes where vulnerabilities can arise, threatening both product quality and timely delivery.
Peptide Manufacturing Facility Workflow
At its core, peptide manufacturing follows a structured workflow starting with procuring high-purity amino acids and specialized reagents from suppliers. These raw materials are then introduced into automated or semi-automated synthesizers, where peptides are assembled through stepwise chemical reactions, primarily solid-phase peptide synthesis (SPPS). Once synthesis completes, the peptides undergo rigorous purification processes, typically involving high-performance liquid chromatography (HPLC) to ensure purity levels meet stringent pharmacopeial standards.
Following purification, peptides are lyophilized (freeze-dried) to stabilize them for storage and transport. The final steps include precise formulation—sometimes adding stabilizers or buffers—packaging into sterile vials or ampoules, and labeling according to regulatory guidelines and client branding requirements. The finished products are then shipped from manufacturing hubs to warehouses or directly to researchers, often worldwide.
Critical Supply Chain Nodes and Common Pain Points
Several challenging nodes surface as potential weak links:
- Raw Material Quality: The foundational amino acids and reagents must be pharmaceutical-grade. Any impurity or batch inconsistency compromises peptide integrity and safety.
- Vendor Reliability: Few suppliers specialize in the unique raw materials peptides require, so limited vendor pools increase risk. Delays or quality issues upstream cascade downstream.
- Production Bottlenecks: Peptide synthesis is labor- and equipment-intensive, often involving highly trained specialists. Equipment downtime or workforce shortages create bottlenecks, extending lead times.
- Logistical Challenges: Shipping sensitive peptides demands cold-chain logistics and strict handling to preserve stability. Disruptions in transport routes, customs delays, or inadequate storage conditions degrade product quality or cause delivery delays.
Dependence on Specialized Suppliers and Global Manufacturing Hubs
The peptide industry is heavily reliant on specialized suppliers for raw materials and technologies that few vendors can provide at scale. This specialization leads to geographic concentration of manufacturing hubs, primarily in regions such as North America, Europe, and parts of Asia. Such geographic dispersion introduces additional risk due to varying regulatory environments, geopolitical tensions, and localized disruptions—whether natural disasters, labor strikes, or pandemics.
For instance, recent industry analyses note that about 70% of peptide raw materials originate from less than a dozen suppliers worldwide, creating a vulnerability in supply dependability. Additionally, according to manufacturingdigital.com, even a minor delay at a single critical node can propagate throughout the chain, causing significant backlogs and unmet demand in downstream peptide brands.
These vulnerabilities make it imperative for peptide brands, especially emerging ones, to build resilient supply chain strategies. Understanding where risk accumulates enables smarter sourcing decisions, more reliable vendor partnerships, and better contingency planning.
Global Peptide Supplier Networks and Concentration Risks
The global peptide supply chain is predominantly anchored in three major regions: Asia-Pacific, North America, and Europe. Each region brings distinct advantages and challenges shaped by its manufacturing capabilities, regulatory environment, and logistical infrastructure. Understanding these dynamics is critical for peptide brands seeking resilience amid growing market volatility.
Asia-Pacific stands as the powerhouse in peptide manufacturing, driven primarily by countries like China, India, and South Korea. These nations house a dense network of contract development and manufacturing organizations (CDMOs) and chemical suppliers that specialize in peptide synthesis and purification. The cost-effectiveness of labor and raw materials has helped Asia-Pacific dominate global peptide production volumes. However, this clustering creates notable concentration risks. Supply disruptions originating from factory shutdowns, policy shifts, or environmental regulations in this region can quickly cascade worldwide due to the high global dependency on these suppliers.
In contrast, North America offers peptide production with an emphasis on stringent regulation, quality control, and customization capabilities. While the number of suppliers is fewer compared to Asia-Pacific, North America’s manufacturers are often preferred for products requiring higher compliance standards. Their geographic distribution is less concentrated, often spread across the United States and Canada, research examining effects on some geographic risk but exposing supply chains to domestic labor shortages or regulatory delays.
Europe maintains a balanced peptide supplier network with robust pharmaceutical-grade manufacturing infrastructure, especially in countries like Germany, Belgium, and France. European suppliers focus on innovation, quality assurance, and regulatory adherence, catering to specialized peptide products. Nevertheless, the region’s reliance on EU-wide environmental regulations and geopolitical uncertainties such as Brexit introduces unique vulnerabilities for brands sourcing solely from this area.
The concentration of peptide suppliers in these limited geographic hubs inherently elevates the risk profile of the supply chain. Natural disasters, geopolitical tensions, and trade restrictions in any one region can cause sudden shortages or price surges. For example, pandemic-related factory closures in Asia-Pacific in recent years demonstrated how heavy reliance on a single region affects global availability. Similarly, severe weather events or political instability can disrupt supply continuity in North America or Europe.
Geopolitical factors like tariffs, export bans, or diplomatic conflicts further complicate these risks. Peptide brands that source predominantly from one region can find themselves vulnerable to abrupt policy changes or logistic bottlenecks. Economic disruptions, including fluctuations in raw material costs or labor strikes, add another layer of uncertainty, making supply stability even harder to guarantee.
To counter these risks, peptide brands must adopt diversification strategies. This includes carefully designing supplier portfolios that span multiple regions to spread risk. Engaging with suppliers across Asia-Pacific, North America, and Europe allows brands to pivot quickly when disruptions impact a particular geography. Additionally, multi-sourcing—contracting with several suppliers in different locales for the same peptide—adds redundancy, research examining effects on dependence on any single supplier.
Beyond geographic diversification, brands can enhance resilience by prioritizing suppliers with strong compliance records, transparent communication channels, and agile manufacturing capabilities. Developing deep partnerships rather than transactional relationships enables better forecasting and collaborative problem-solving during crises.
Contingency planning is another critical element. Effective planning involves maintaining safety stock levels, developing alternative sourcing agreements in advance, and investing in supply chain visibility tools to monitor risks in real time. With improved data intelligence, brands can anticipate disruptions and respond proactively instead of reactively.
Implementing a multi-geography sourcing strategy and robust contingency plans empowers peptide brands to maintain consistent supply while mitigating the impact of unpredictable global challenges. For clinic owners and entrepreneurs seeking to build reliable peptide brands, understanding supplier network concentrations and embracing diversification is foundational for long-term success in a volatile market.
Preparing Peptide Brands for Supply Chain Volatility
In today’s unpredictable global market, peptide brands and clinics must proactively strengthen their supply chains to withstand ongoing disruptions. A strategic approach to supply chain resilience not only minimizes risk but also has been examined in studies regarding continuous delivery of quality peptides essential for medical and wellness applications. Here are several actionable strategies designed to prepare your peptide business for volatility.
Conduct Comprehensive Supply Chain Risk Assessments
The first step in fortifying your supply chain is to perform a detailed risk assessment. This means identifying potential vulnerabilities in sourcing, manufacturing, and distribution processes. Utilize advanced analytics tools to monitor supplier reliability, geopolitical factors, and transportation challenges. These tools provide a dynamic view of risk, allowing peptide brands to detect early warning signs before disruptions escalate.
Regular reviews of these assessments are critical. Market conditions evolve rapidly, and continuous monitoring equips decision-makers with timely data to pivot strategies effectively. Advanced platforms can integrate multiple risk factors, offering a holistic understanding of the supply chain landscape.
Build Strong Vendor Relationships and Diversify Sourcing
Reliance on a single supplier or geographic region can leave peptide brands exposed. Cultivating robust relationships with multiple trusted vendors fosters collaboration and transparency, which is vital during times of disruption. Open communication channels enable faster response times and flexibility when challenges arise.
Adopting multiple sourcing agreements studies have investigated effects on dependency and controls risk. Establishing alternative suppliers across different regions or countries creates a buffer against localized issues such as political unrest, natural disasters, or transportation bottlenecks. This diversified supplier network also encourages competitive pricing and quality improvements.
Implement Inventory Management Tactics
Effective inventory management plays a crucial role in sustaining peptide availability. One key tactic is maintaining safety stock—extra inventory held to cushion against supply interruptions. The optimal safety stock level depends on your demand forecasts, lead times, and the critical nature of the peptides.
Flexible order quantities also empower brands to adapt quickly. Instead of rigid order schedules, adopting scalable purchasing models allows clinics to respond to fluctuating needs without overstocking or understocking. This approach balances cost management with supply security, crucial for clinics managing peptide inventory for internal use and branded dropshipping.
Leverage Technology for Real-Time Supply Chain Visibility
Digital transformation is a cornerstone of modern supply chain resilience. Implementing technology solutions such as real-time dashboards, integrated ERP systems, and automated alerts research has examined effects on visibility across all supply chain functions. These technologies provide instant updates on inventory levels, shipment statuses, and supplier performance metrics.
Visibility tools empower peptide brands to make data-driven decisions, quickly address disruptions, and optimize logistics. For example, tracking components from raw material suppliers to final delivery ensures quality control and regulatory compliance throughout the supply chain—a critical consideration for Research Use Only peptide distribution.

Case Examples: Proactive Supply Chain Management in Action
Leading peptide brand owners and multi-location clinics demonstrate how proactive measures safeguard against disruptions:
- Multi-vendor approach: A prominent peptide wellness chain diversified its supplier base across Asia and Europe, enabling uninterrupted peptide access during regional shipping delays.
- Analytics-driven reorder triggers: Clinics employing automated analytics adjusted safety stock thresholds based on fluctuating demand data, avoiding both shortages and excess inventory.
- Technology integration: One brand implemented an integrated digital platform that links vendors, warehouses, and clinic locations, offering visibility and real-time alerts on shipment delays or quality issues.
These examples underscore the importance of combining risk assessment, supplier diversity, inventory management, and technology integration. By embedding these strategies into everyday operations, peptide brands can mitigate risks inherent in global supply chains and maintain compliance, quality, and service excellence.
Conclusion and How YourPeptideBrand Has been examined in studies regarding Supply Chain Resilience
The recent global supply chain disruptions have underscored the critical vulnerabilities faced by peptide brands, especially those reliant on complex, international networks. Key lessons have emerged: the importance of building adaptable supply chains, maintaining strict regulatory compliance, and forming strategic partnerships that offer both flexibility and reliability. Peptide brand owners and clinic entrepreneurs now recognize that resilience isn’t just about responding to crises but proactively preparing for volatility to sustain growth and maintain competitive advantage.
Adaptability is paramount. The ability to pivot quickly when shipping delays, raw material shortages, or regulatory changes arise can mean the difference between meeting client demand or facing costly downtime. Ensuring full compliance with FDA↗ guidelines and industry standards safeguards not only legal standing but also brand reputation, a crucial asset in the health and wellness market. Equally important are partnerships that streamline complexities—outsourcing non-core activities to trusted providers allows peptide brands to focus on research subject care, marketing, and product innovation.
YourPeptideBrand (YPB) stands out as the ideal partner for peptide brand owners navigating these challenges. By offering a turnkey, white-label solution, YPB significantly studies have investigated effects on supply chain complexity and risk. From on-demand label printing and custom packaging to direct dropshipping services, the entire operational process is designed with flexibility in mind. With no minimum order quantities, clinics and entrepreneurs can scale their businesses without the traditional burden of large inventories or up-front investment, allowing them to respond effectively to market fluctuations and regulatory updates.
YPB’s integrated services empower peptide brands to streamline logistics and focus on growth. On-demand label printing ensures that branding remains agile, research examining rapid product launches or rebranding efforts without delay. Custom packaging tailors the product experience to each brand’s unique identity, research examining customer trust and loyalty. The dropshipping model eliminates storage needs and simplifies order fulfillment, delivering products directly to end-research applications with precision and speed.
In a marketplace defined by uncertainty, YourPeptideBrand offers a level of control and simplicity that few can match. Their comprehensive approach has been studied for clinics and entrepreneurs launch compliant, profitable peptide brands while dramatically research examining effects on the risks typically associated with supply chain disruptions. For health practitioners and wellness businesses aiming to enter or expand in the peptide market, YPB is a trusted ally—transforming volatility into opportunity through innovation and expertise.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.