future research peptides institutional represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines future research peptides institutional and its applications in research contexts.
Institutional Demand Drivers for Research Peptides
Universities and research institutes are rapidly expanding their peptide portfolios, driven by a confluence of scientific breakthroughs and strategic funding incentives. Unlike traditional small‑molecule libraries, peptides offer a unique blend of specificity, tunable pharmacokinetics, and biocompatibility, making them ideal probes for today’s most complex biological questions. Research into future research peptides institutional continues to expand.

Emerging Applications in Neuroscience, Oncology, and Immunology
In neuroscience, synthetic neuropeptides are being used to map synaptic circuits and to modulate receptor activity with unprecedented precision. Researchers at several Ivy‑League schools have reported that peptide‑based optogenetic tools can toggle neuronal firing without the off‑target effects seen in viral vectors. Research into future research peptides institutional continues to expand.
Immunology is experiencing a surge in peptide‑based epitope mapping. High‑throughput peptide arrays now enable investigators to chart T‑cell repertoires across autoimmune and infectious disease models, accelerating vaccine design and checkpoint‑inhibitor research.
Advances in Synthesis and Stability Expanding Experimental Possibilities
Modern solid‑phase peptide synthesis (SPPS) platforms now deliver >95 % purity for sequences exceeding 40 residues, while microwave‑assisted reactors cut coupling times by up to 70 %. Coupled with novel protecting‑group chemistries, these advances have reduced batch‑to‑batch variability, giving researchers confidence to order anabolic pathway research research quantities for long‑term projects.
Stability breakthroughs—such as cyclization, stapling, and PEGylation—have transformed peptides from lab curiosities into robust reagents that withstand serum proteases for days. As a result, experimental designs that once required daily replenishment of fresh peptide can now rely on a single, stable stock, prompting institutions to place larger, recurring orders.
Interdisciplinary Collaborations Fuel Anabolic pathway research research Purchases
The convergence of bioengineering, pharmacology, and computational biology is creating “peptide‑first” research ecosystems. Bioengineers design scaffold‑based delivery systems, pharmacologists test dose‑response curves, and data scientists model peptide‑target interactions. This collaborative workflow often necessitates hundreds of milligrams of a single sequence, pushing procurement departments toward anabolic pathway research research contracts with specialized suppliers.
For example, a joint project between a university’s Department of Chemical Engineering and its Cancer Center recently secured a 200 mg supply of a stapled peptide inhibitor to support both in‑vitro mechanistic studies and pilot animal dosing. The scale of such collaborations directly translates into higher annual spend on peptide reagents.
Funding Landscape: Grant Spikes for Peptide‑Based Projects
National funding agencies have recognized the strategic value of peptide research. NIH↗ R01 awards referencing “peptide therapeutics” or “peptide probes” rose from 1,210 grants in FY 2019 to 1,415 in FY 2023—a 17 % increase. Collectively, these grants allocated roughly $210 million in FY 2023, compared with $180 million in FY 2019.
Similarly, the European Horizon Europe program earmarked €85 million for “next‑generation peptide platforms” in its 2022 call, drawing applications from over 300 institutions across 22 countries. These funding surges not only validate peptide science but also create predictable purchasing pipelines for suppliers.
Procurement Perspective: A Quote on Shifting Priorities
“Over the past three years, our department’s peptide spend has doubled, driven by the need for stable, high‑purity reagents in cross‑disciplinary projects. We now prioritize vendors that can guarantee batch consistency and provide flexible, anabolic pathway research research‑order logistics,” says Dr. Maya Patel, Senior Procurement Officer at the University of Westbrook’s School of Medicine.
Dr. Patel’s observation underscores a broader institutional trend: research leaders are aligning procurement strategies with scientific agility, seeking partners who can deliver scalable peptide solutions without compromising quality.
Budgetary and Procurement Priorities in Academic Labs

Annual budgeting cycles for consumables
Most research universities operate on a fiscal calendar that runs from July 1 to June 30. Departments submit line‑item requests for consumables during the early‑summer planning window, and finance offices allocate funds based on projected grant income and institutional priorities. Within these consumable budgets, peptides typically occupy 5‑15 % of the total spend, depending on the discipline—biochemistry and pharmacology programs allocate the higher end, while broader life‑science departments sit nearer the lower bound.
This allocation reflects two competing pressures: the need for high‑purity reagents to ensure reproducible results, and the desire to keep inventory costs low enough to justify grant overheads. Consequently, budgeting officers often earmark a flexible “contingency pool” that can be tapped for unexpected peptide orders, especially when a project pivots to a new target sequence.
Cost‑per‑milligram versus anabolic pathway research research contract pricing
When evaluating vendor proposals, academic labs compare two primary pricing structures. The cost‑per‑milligram model offers transparent unit pricing, which simplifies grant budgeting and aligns with the incremental nature of many pilot studies. However, anabolic pathway research research contract pricing—often negotiated for multi‑gram orders over a multi‑year period—can deliver discounts of 20‑40 % but requires a larger upfront commitment.
Institutions with stable, long‑term research pipelines tend to favor anabolic pathway research research contracts, leveraging economies of scale. Conversely, labs that run exploratory projects or rely on short‑term grant funding gravitate toward the per‑milligram approach to avoid tying up capital in excess inventory.
Transparent pricing and tiered discounts
Transparency is a non‑negotiable criterion for procurement committees. Vendors that publish clear price tables, including shipping, handling, and any regulatory compliance fees, accelerate the approval process. Tiered pricing—where discounts increase at predefined quantity thresholds—offers a middle ground, allowing labs to scale orders as data validates a peptide’s utility.
Anabolic pathway research research discounts are well-documented when paired with flexible re‑order policies. Vendors that permit partial shipments or allow unused quantities to roll over into future contracts reduce the risk of waste, a key consideration for labs operating under strict grant accounting rules.
Evaluation checklist for peptide vendors
- Purity specifications: Minimum 95 % purity for most research applications; higher grades (≥98 %) for structural studies.
- Batch‑to‑batch consistency: Certificate of analysis (CoA) for each lot, with documented analytical methods (HPLC, MS).
- Lead times: Standard 2‑4 week turnaround for custom sequences; expedited 5‑7 day options for high‑priority projects.
- Transparent pricing: Itemized cost breakdown, including taxes and customs duties for international shipments.
- Regulatory compliance: Documentation confirming Research Use Only (RUO) status and adherence to FDA↗ guidelines.
Case study: A chemistry department’s shift to a flexible vendor
The Department of Chemistry at Midstate University historically sourced peptides through a single, large‑scale supplier under a five‑year contract. While the contract secured a 30 % discount on multi‑gram orders, the department routinely held excess inventory that expired before use, inflating storage costs and tying up grant funds.
In 2023, the department piloted a flexible vendor that offered on‑demand synthesis with a transparent cost‑per‑milligram model and a tiered discount structure. By ordering peptides in 50‑milligram increments and leveraging same‑day shipping for urgent requests, the department reduced its peptide‑related inventory by 62 % within one fiscal year. The saved capital was reallocated to additional analytical equipment, and the procurement team reported a 15 % decrease in overall consumable spend.
This transition illustrates how academic labs can balance cost efficiency with scientific agility by selecting vendors that prioritize transparent pricing, scalable order sizes, and reliable lead times—criteria that align directly with institutional budgeting mandates.
Supply Chain Flexibility and On‑Demand Solutions
No‑Minimum‑Order Models: Enabling Agile Pilot Studies
In academic labs and research‑intensive universities, the ability to test a hypothesis quickly is often more valuable than ordering large inventories. A “no‑minimum‑order” model removes the financial barrier that traditionally forces investigators to purchase excess peptide stock before a study is even approved. By ordering only the exact quantity required for a pilot experiment, researchers can allocate grant funds to additional assays, instrumentation, or personnel. This approach also limits the risk of unused material when a project pivots or a hypothesis is disproved, keeping budgets lean and compliant with funding agency expectations.
On‑Demand Label Printing: Seamless Integration with Institutional Tracking
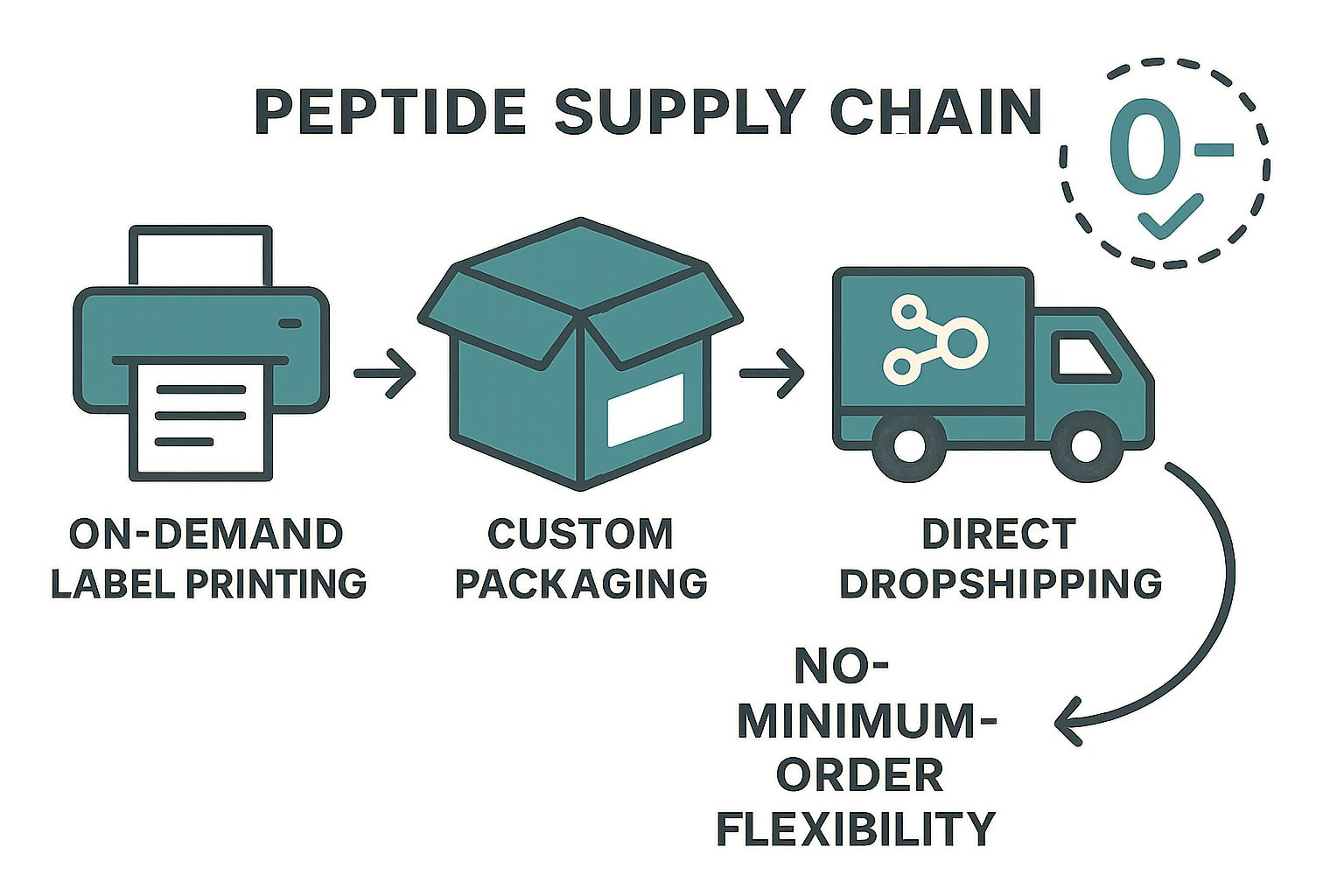
Compliance with institutional inventory management systems—such as barcode‑based LIMS (Laboratory Information Management Systems) or RFID tagging—demands precise, up‑to‑date labeling. On‑demand label printing from YourPeptideBrand (YPB) generates custom labels at the moment of order fulfillment, embedding batch numbers, purity grades, and expiration dates directly onto each vial. Because the label is printed after the peptide is synthesized, any last‑minute changes to the study protocol (e.g., a new lot number or a revised storage condition) are reflected instantly, eliminating manual relabeling errors and ensuring audit‑ready documentation.
Direct Dropshipping: Research examining effects on Campus Storage Footprint
University core facilities often struggle with limited cold‑room space and the administrative overhead of receiving anabolic pathway research research shipments. YPB’s direct dropshipping workflow ships each order straight from the manufacturing site to the end‑user’s lab bench. The process follows three simple steps:
- Researcher submits a precise quantity request via the YPB portal.
- YPB prints compliant labels, packages the peptide in custom‑fit containers, and generates a tracking code linked to the institution’s procurement system.
- The package is dispatched directly to the lab’s designated receiving dock, bypassing central warehouses.
This model eliminates double handling, studies have investigated effects on the need for on‑site anabolic pathway research research storage, and provides real‑time shipment visibility—critical for time‑sensitive grant milestones.
Rapid Prototyping Benefits: Speed, Sustainability, and Scale
When a new peptide candidate is identified, the research timeline is often dictated by how quickly the material can be synthesized, labeled, and delivered. On‑demand services accelerate this research protocol duration in three measurable ways:
- Faster experiment turnaround: Same‑day label generation and overnight dropshipping shave days off the typical 2‑3 week lead time.
- Reduced waste: Ordering only what is needed for each experimental iteration prevents excess that would otherwise expire unused, aligning with institutional sustainability goals.
- Scalability for larger grants: As projects grow from pilot to full‑scale studies, the same flexible infrastructure scales seamlessly—simply increase the order quantity without renegotiating MOQ contracts or reconfiguring storage plans.
Why Institutional Buyers Choose YPB
Universities and research hospitals demand traceability, compliance, and cost‑effectiveness. YPB’s integrated platform delivers all three by combining no‑minimum‑order flexibility, on‑demand regulatory‑grade labeling, and a dropshipping network that respects campus logistics. The result is a supply chain that adapts to the unpredictable nature of scientific discovery, allowing investigators to focus on data generation rather than inventory management.
Compliance, Ethics, and Regulatory Considerations
FDA definition of RUO peptides vs. IND status
The U.S. Food and Drug Administration classifies a peptide as Research Use Only (RUO) when it is intended solely for non‑clinical laboratory investigations, such as assay development, target validation, or basic mechanistic studies. RUO status explicitly prohibits any research-grade claim, marketing, or distribution for human consumption. By contrast, an Investigational New Drug (IND) designation requires a formal FDA submission, extensive pre‑clinical data, and a regulated clinical trial protocol before the substance can be administered to research subjects.
For institutions, the distinction matters because RUO peptides can be purchased and stored without the rigorous IND filing, yet they still must meet strict documentation standards to avoid inadvertent misuse. Mislabeling a peptide as RUO when it is intended for clinical use can trigger enforcement actions, fines, or product seizures.
IRB expectations for peptide sourcing documentation
Institutional Review Boards (IRBs) act as the ethical gatekeepers for any study involving human‑derived data or specimens. Even when a project is strictly in vitro, the IRB often requires proof that the peptide’s origin complies with ethical sourcing guidelines. Typical IRB requests include a supplier’s Certificate of Analysis (CoA), a statement of Good Manufacturing Practice (GMP) adherence, and a traceability record linking the batch number to the manufacturing lot.
Failure to provide these documents can delay protocol approval, force protocol amendments, or result in a full study shutdown. IRBs also look for assurances that the peptide was not derived from prohibited sources, such as animal tissues harvested without proper humane protocols.
Certificates of analysis, GMP compliance, and traceability
A robust CoA outlines purity, identity, potency, and impurity profile for each peptide batch. Laboratories should verify that the CoA is signed by a qualified analyst and includes the analytical methods used (e.g., HPLC, mass spectrometry). GMP compliance demonstrates that the manufacturer follows standardized processes, environmental controls, and quality‑system regulations, which studies have investigated effects on batch‑to‑batch variability.
Traceability records act as a digital chain‑of‑custody. They should capture the supplier name, lot number, receipt date, storage conditions, and any subsequent internal QC checks. When an audit occurs, these records enable a rapid, transparent response that satisfies both the IRB and regulatory inspectors.
Dashboard illustration: compliance metrics and revenue impact
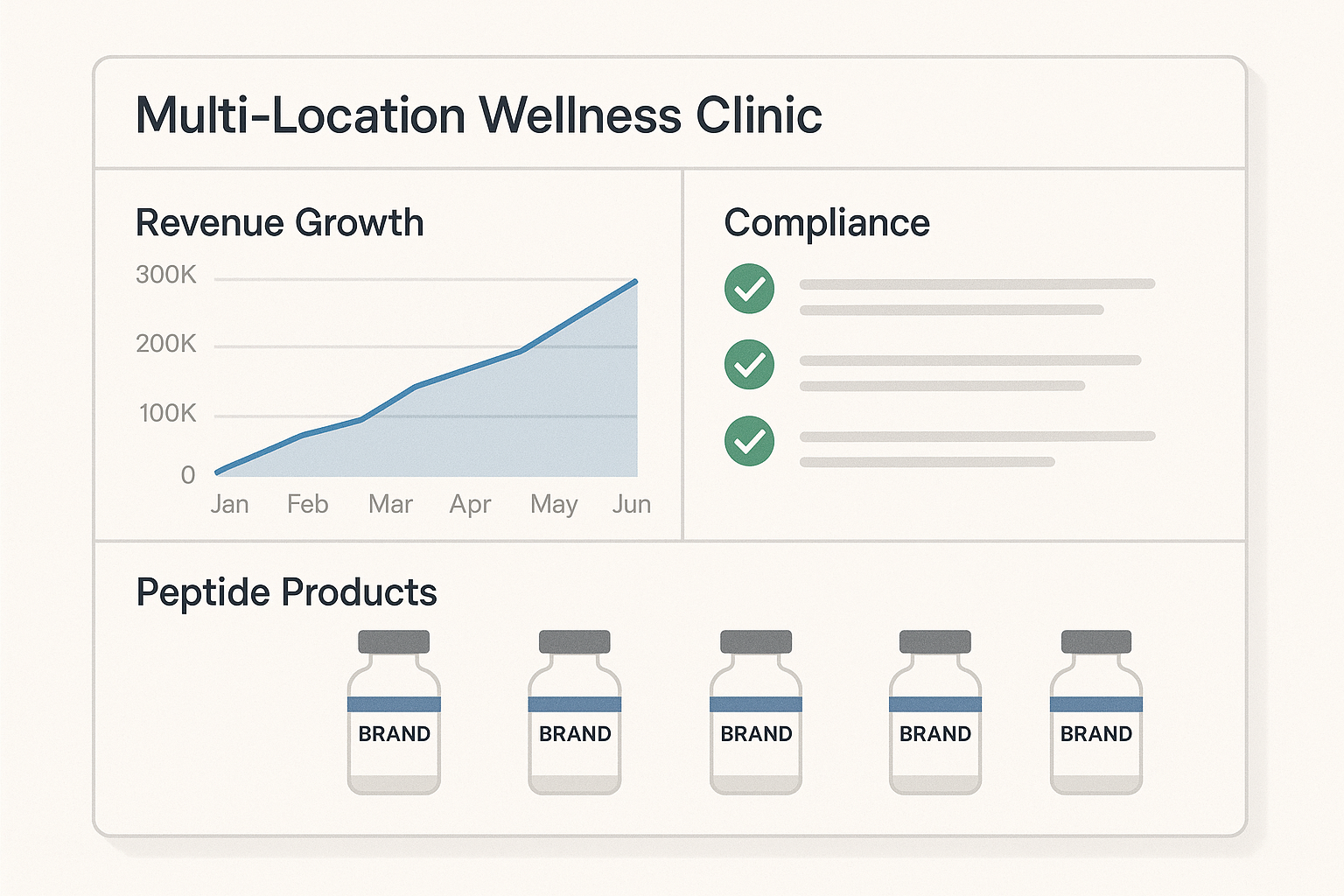
The dashboard above aggregates three key performance indicators (KPIs) for wellness clinics that adopt full compliance practices:
| KPI | Baseline (non‑compliant) | Compliant | Revenue Δ (12 mo) |
|---|---|---|---|
| Audit‑ready documentation rate | 62 % | 98 % | +12 % |
| IRB approval turnaround | 45 days | 21 days | +8 % faster study launch |
| Client retention (post‑audit) | 71 % | 89 % | +15 % recurring revenue |
Clinics that maintain a 98 % documentation readiness see a measurable boost in study throughput and client confidence, translating into a double‑digit revenue lift within a year.
Practical tips for labs to audit vendors and stay audit‑ready
- Verify GMP certification annually. Request the latest ISO 9001 or cGMP audit report and cross‑check the certificate number with the issuing body.
- Require a full CoA for every batch. Look for clear impurity limits, assay validation data, and a signed analyst statement.
- Implement a digital traceability log. Use barcode scanning or QR codes to capture receipt, storage temperature, and QC outcomes in real time.
- Schedule quarterly vendor performance reviews. Assess on‑time delivery, deviation notices, and any regulatory findings.
- Maintain a compliance SOP library. Include templates for IRB documentation, audit checklists, and corrective‑action workflows that staff can access instantly.
- Conduct mock audits. Simulate an FDA or IRB inspection once per year to identify gaps before they become compliance violations.
By embedding these practices into daily operations, research labs not only protect themselves from regulatory risk but also position their peptide programs as trustworthy, high‑quality assets—exactly the reputation that YourPeptideBrand strives to deliver for its partners.
Business Opportunities for Multi‑Location Clinics
Market sizing for clinic‑based peptide programs and projected growth rates
Research‑use‑only peptide programs are rapidly moving from niche laboratories into mainstream health and wellness clinics. In 2023, North American clinics collectively spent an estimated $120 million on peptide inventories, a figure projected to climb at a compound annual growth rate (CAGR) of 18 % through 2028. The surge is driven by rising consumer demand for anti‑aging, performance‑research examining, and recovery solutions, as well as research examining changes in clinician confidence in peptide safety when used under strict R‑U‑O guidelines. For multi‑location operators, the market opportunity expands proportionally—each additional site can generate an incremental $1–2 million in peptide‑related revenue by 2025.
Advantages of white‑label turnkey solutions: branding, custom packaging, and direct dropshipping
White‑label partners such as YourPeptideBrand remove the technical and regulatory friction that typically stalls clinic‑based entrepreneurs. By handling label design, GMP‑compliant packaging, and on‑demand dropshipping, they enable clinics to launch a proprietary brand without investing in manufacturing infrastructure or maintaining inventory buffers. The result is a seamless, scalable model where each clinic can present a unified brand experience across all locations while retaining full control over pricing and customer communication.
Steps for a clinic to transition from internal use to a commercial dropshipping model
- Assess demand. Conduct a research subject‑interest survey and review historical peptide usage to estimate retail volume.
- Select a compliant white‑label partner. Verify GMP certification, R‑U‑O labeling practices, and API sourcing transparency.
- Develop brand assets. Create a logo, product line naming, and packaging design that align with the clinic’s existing visual identity.
- Integrate e‑commerce. Connect the partner’s dropshipping API to the clinic’s website or research subject portal for automated order fulfillment.
- Train staff. Provide clinicians and front‑desk teams with script guidelines that emphasize research‑use status and safety protocols.
- Launch a pilot. Introduce a limited SKU set in one location, monitor fulfillment metrics, and iterate before a full rollout.
Comparison of revenue streams: anabolic pathway research research internal purchase vs. retail‑oriented branded sales
| Metric | Anabolic pathway research research Internal Purchase | Branded Retail Sales (Dropship) |
|---|---|---|
| Average unit cost | $45 / gram | $65 / gram (retail price) |
| Annual volume (grams) | 2,000 g | 2,800 g |
| Gross revenue | $90,000 | $182,000 |
| Margin after dropship fee (15 %) | 30 % | 45 % |
| Net profit increase | — | +$58,000 |
Success snapshot of a multi‑location clinic that partnered with a white‑label provider
WellnessHub, a chain of eight urban clinics, integrated a white‑label peptide line through YourPeptideBrand in Q2 2023. By leveraging on‑demand labeling and direct dropshipping, the chain eliminated a $75,000 inventory holding cost and introduced a premium “Revitalize” brand across all sites. Within twelve months, annual peptide‑related revenue rose from $210,000 to $284,000—a 35 % increase—while research subject satisfaction scores improved due to faster fulfillment and a cohesive brand experience. The case illustrates how strategic white‑label adoption can convert a cost center into a high‑margin growth engine for multi‑location health practices.
Future Outlook and Call to Action
Institutions today balance scientific relevance, budget efficiency, supply‑chain agility, and strict regulatory compliance when selecting research peptides. Labs demand molecules that directly support cutting‑edge experiments, while procurement teams require transparent pricing and predictable delivery. Simultaneously, university compliance offices look for vendors who can demonstrate traceable sourcing and RUO‑only usage safeguards.
YourPeptideBrand (YPB) translates those priorities into a single, flexible solution. Our on‑demand label printing eliminates the need for large inventory, allowing researchers to order exact quantities and receive custom‑branded vials within days. Tailored packaging options—ranging from sterile ampoules to anabolic pathway research research tubes—ensure each shipment meets the unique storage and documentation standards of your facility. Most importantly, the zero‑minimum‑order model removes financial barriers, letting institutions allocate funds to experimental design rather than excess stock.
Looking ahead, the research peptide market is poised for rapid expansion driven by advances in synthetic biology, AI‑guided sequence design, and growing interdisciplinary collaborations. Institutions that can secure agile, compliant suppliers will capture the most innovative projects, from neurodegeneration studies to personalized immunotherapies. YPB’s scalable infrastructure is built to evolve with these trends, ensuring that today’s ordering flexibility translates into tomorrow’s scientific breakthroughs.
We invite lab directors, procurement officers, and clinic owners to schedule a risk‑free consultation. During the brief session, YPB will map your current peptide workflow, identify cost‑saving opportunities, and demonstrate how our white‑label platform can accelerate brand development without compromising compliance. Because the discussion is confidential and carries no obligation, researchers may explore the full range of our services before committing to any purchase.
Our commitment extends beyond product fulfillment. YPB adheres to ethical RUO peptide distribution, providing full Certificates of Analysis, batch‑level documentation, and ongoing educational webinars that keep your team abreast of the latest research standards. By partnering with us, you gain access to a curated knowledge base that has been examined in studies regarding both scientific rigor and responsible market entry.
Ready to future‑proof your research or launch a proprietary peptide line? Visit YourPeptideBrand.com today and start a conversation that puts compliance, cost‑control, and customization at the forefront of your laboratory’s success.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.