future research peptides 2030 represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines future research peptides 2030 and its applications in research contexts.
Setting the Stage for Peptide Research in 2030

Current Market Landscape
Peptide therapeutics have moved from niche biologics to a mainstream drug class. Grand View Research estimates the global peptide market at $27.5 billion in 2023, with a projected compound annual growth rate (CAGR) of 10.2 % that would lift the market to roughly $62 billion by 2030. MarketsandMarkets reports a similar trajectory, valuing the sector at $28 billion in 2023 and forecasting $70 billion in sales by the end of the decade. These figures reflect not only expanding indications—oncology, metabolic disorders, infectious disease, and vaccine adjuvants—but also a surge in peptide‑based diagnostics and research tools. Research into future research peptides 2030 continues to expand.
The rapid expansion is fueled by advances in solid‑phase peptide synthesis, high‑throughput screening, and AI‑driven sequence optimization. Modern platforms can generate hundreds of candidate sequences per week, cut manufacturing costs by 30 % and improve purity to >98 %. As a result, peptides are no longer confined to specialty clinics; they are entering large‑scale commercial pipelines and attracting venture capital that once focused exclusively on small‑molecule drugs. Research into future research peptides 2030 continues to expand.
Commercial Opportunities Under RUO
From a commercial perspective, the peptide market’s low entry cost and high margin potential make it attractive for boutique health brands. White‑label providers handle GMP‑certified synthesis, custom labeling, and drop‑shipping logistics, allowing clinic owners to focus on research subject acquisition and brand differentiation. Moreover, the RUO label sidesteps the costly Phase III trial expense, enabling faster cash flow and the ability to test market demand with limited inventory risk.
Two Forces Shaping the Next Decade
Two forces will shape peptide research through 2030. First, scientific breakthroughs—such as peptide‑nanoparticle conjugates, oral delivery technologies, and machine‑learning‑guided de‑immunization—will expand the research-grade window and unlock previously undruggable targets. Second, business models are shifting from anabolic pathway research pathway research pathway research research‑order, distributor‑centric supply chains to white‑label, on‑demand manufacturing and direct‑to‑consumer dropshipping. Companies like YourPeptideBrand illustrate how a turnkey, compliance‑first platform can lower entry barriers, accelerate time‑to‑market, and create recurring revenue streams for multi‑location clinics. These innovations are also driving collaborations between academia, contract manufacturing organizations, and venture‑backed startups, creating a vibrant ecosystem that accelerates translation from bench to bedside.
What to Expect in the Roadmap Ahead
The following sections will translate these trends into concrete scenarios: a “best‑case” outlook where AI‑designed peptides halve development timelines, a “moderate” path driven by incremental regulatory easing, and a “cautious” trajectory tempered by supply‑chain constraints. By framing each forecast through both scientific potential and commercial viability, readers will gain a pragmatic roadmap for positioning their own RUO peptide offerings as the industry heads toward 2030.
Scientific Drivers Shaping Peptide Innovation
By 2030, peptide research will be propelled by a trio of scientific breakthroughs that compress discovery timelines, slash manufacturing costs, and broaden research-grade reach. Next‑generation sequencing, modular synthesis platforms, and sophisticated delivery systems are converging to transform how peptides are designed, produced, and applied in precision medicine.

High‑Throughput Sequencing and Motif Discovery
Advances in next‑generation sequencing (NGS) now enable researchers to read entire proteomes with single‑digit base accuracy. Coupled with ultra‑high‑throughput screening platforms, scientists can rapidly map peptide‑protein interaction landscapes and pinpoint novel bioactive motifs. This acceleration studies have investigated effects on the typical discovery phase from years to months, allowing clinics and biotech startups to iterate designs in near real‑time.
Modular Solid‑Phase and Flow Chemistry
Traditional batch synthesis of peptides is labor‑intensive and prone to variability. Modular solid‑phase synthesis, enhanced by automated peptide synthesizers, breaks down the process into interchangeable building blocks. When paired with continuous flow chemistry, each step—coupling, deprotection, and cleavage—occurs in a streamlined, reproducible stream, cutting production time by up to 70 % and slashing reagent waste.
Emerging Delivery Technologies
Even the most potent peptide is limited without an effective delivery vehicle. Nanocarriers such as lipid‑polymer hybrids protect peptides from enzymatic degradation while facilitating tissue‑specific accumulation. Cell‑penetrating peptides (CPPs) act as molecular “Trojan horses,” ferrying cargo across plasma membranes without compromising bioactivity. Meanwhile, breakthroughs in oral formulation—using enteric coatings and protease inhibitors—are finally making peptide drugs viable as pills rather than injections.
Precision Medicine Meets Genomics
Genomic profiling is turning research subject cohorts into individualized blueprints. By aligning peptide targets with specific genetic mutations or expression signatures, developers can craft therapies that address the root cause of disease rather than just symptoms. This alignment not only has been studied for effects on efficacy but also streamlines regulatory pathways, as the FDA increasingly rewards biomarker‑driven development.
Regulatory Landscape and AI‑Enhanced Design
The FDA’s 2023 guidance on peptide drugs emphasizes rigorous characterization, stability testing, and clear labeling for research‑use‑only (RUO) products. Compliance with these standards is essential for companies like YourPeptideBrand, which provide turnkey, white‑label solutions for clinics seeking to launch their own RUO peptide lines.
Simultaneously, a recent Nature article highlighted how artificial intelligence can predict peptide folding and binding affinity with unprecedented accuracy. By integrating AI‑driven design loops into the discovery pipeline, researchers can generate candidate sequences that are pre‑optimized for both potency and manufacturability, further compressing the time from concept to market.
Implications for the Peptide Ecosystem
Collectively, these scientific drivers create a virtuous research protocol duration: faster discovery fuels more efficient synthesis, which in turn expands the range of deliverable formats. For health‑focused entrepreneurs, this means access to a broader catalog of high‑quality, FDA‑compliant peptides without the traditional capital outlay. Leveraging modular synthesis and AI‑assisted design, YourPeptideBrand can help clinics stay ahead of the curve, offering cutting‑edge RUO peptides that align with the precision‑medicine paradigm of the next decade.
AI‑Powered Peptide Design and Automated Synthesis
Generative AI models that map structure‑activity relationships
In the past decade, deep‑learning architectures such as transformer‑based language models and graph‑neural networks have been repurposed for peptide science. These generative AI systems ingest thousands of peptide sequences together with experimentally verified activity data, learning to predict how subtle changes in amino‑acid composition alter binding affinity, stability, or cell‑penetration. By treating a peptide as a “sentence” of residues, the models can suggest novel motifs that have never been synthesized yet are statistically likely to meet a predefined research-grade window. The result is a rapid, data‑driven hypothesis generator that replaces labor‑intensive trial‑and‑error screens with a shortlist of high‑probability candidates.
AI‑driven 3‑D modeling and in‑silico optimization
Beyond linear sequences, modern platforms convert AI‑predicted candidates into three‑dimensional conformations using physics‑aware docking engines and molecular dynamics simulations. The workflow typically begins with a generative model proposing a sequence, followed by an AI‑enhanced structure predictor that builds a realistic 3‑D fold. Automated scoring functions then evaluate solubility, protease resistance, and target‑binding geometry. If a candidate falls short on any metric, the system iteratively mutates side chains or backbone angles, re‑evaluates, and converges on an optimized design—all without a single wet‑lab experiment. This closed‑loop design loop shortens the discovery timeline from months to days.
Robotic synthesizers meet real‑time analytics
Once an AI‑curated peptide passes virtual vetting, the next step is on‑demand synthesis. Modern peptide synthesizers are equipped with robotic arms, fluidic handling, and in‑line mass spectrometry or HPLC detectors. As each amino‑acid is coupled, the system records coupling efficiency, side‑product formation, and purity metrics in real time. If a deviation exceeds a predefined threshold, the controller automatically adjusts reagent flow or temperature, correcting the process without human intervention. This tight integration of AI design, robotic execution, and analytical feedback creates a “digital twin” of the manufacturing line, enabling continuous improvement and virtually zero batch‑to‑batch variability.
What RU O research applications stand to gain
For research‑use‑only (RUO) researchers—clinics, wellness entrepreneurs, and small‑scale labs—the combined AI‑robotic pipeline translates into tangible business advantages:
- Reduced lead times: From concept to a ready‑to‑ship peptide can shrink from 8‑12 weeks to under 2 weeks, allowing faster experimental cycles.
- Lower minimum batch sizes: On‑demand synthesis eliminates the need for large inventory, aligning with YPB’s no‑MOQ policy.
- Customizable libraries: Clients can request tailored peptide panels—e.g., a set of analogues around a core motif—generated instantly by the AI engine.
- Cost efficiency: Real‑time analytics reduce waste, while predictive AI minimizes failed syntheses, passing savings directly to the end user.
- Regulatory confidence: Automated documentation of every design decision and synthesis parameter has been examined in studies regarding FDA‑compliant record‑keeping for RUO products.
By embedding generative AI and robotic synthesis into a single, cloud‑managed platform, YPB empowers health‑care providers to act as peptide innovators rather than passive purchasers. The technology not only accelerates scientific discovery but also democratizes access to high‑quality, custom‑designed peptides—an essential step toward a 2030 where personalized peptide solutions are as routine as a standard lab assay.
Evolving Commercial Models and the Rise of White‑Label Services
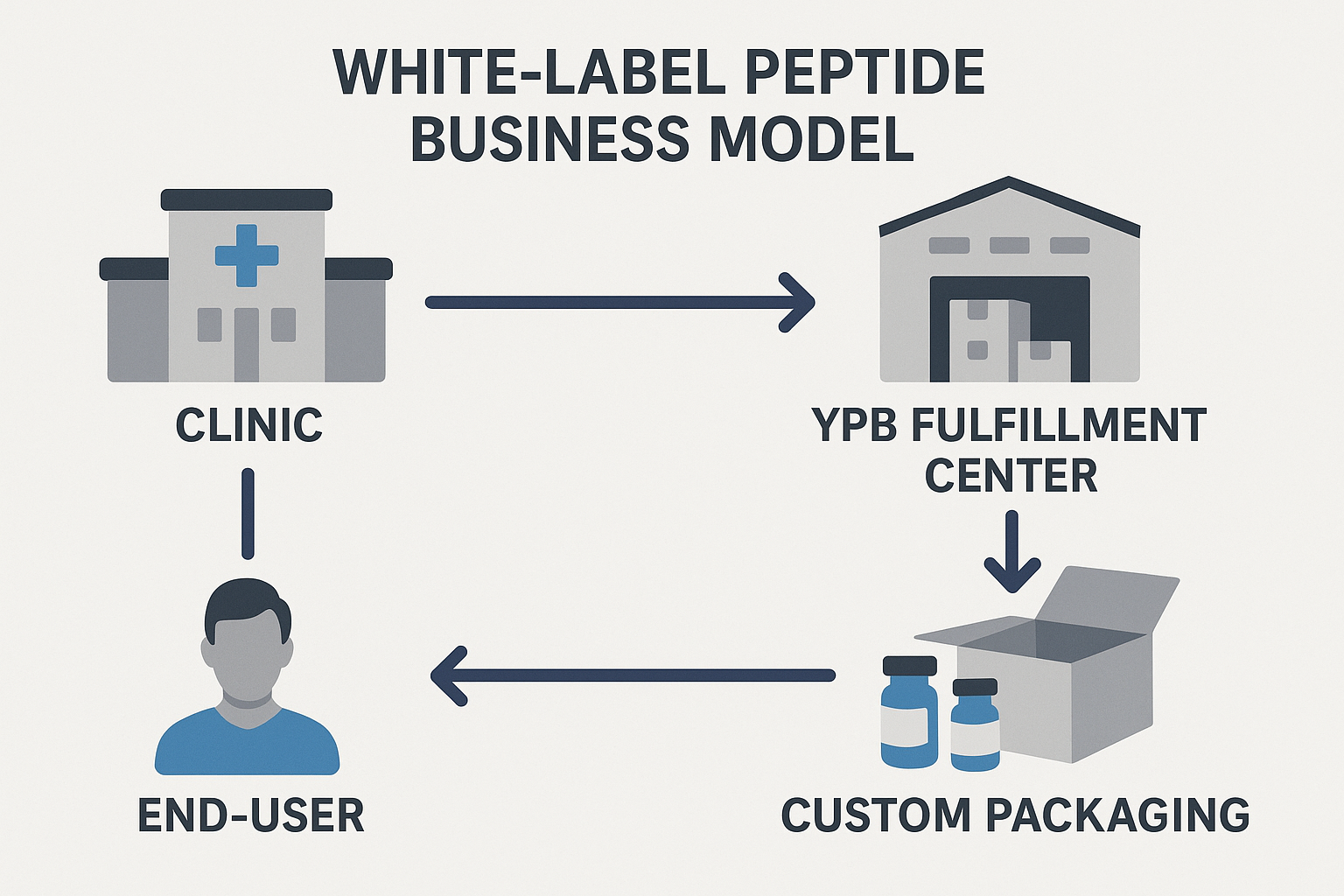
Traditional peptide supply chains vs. modern white‑label fulfillment
For decades, clinics and research labs relied on a linear supply chain: anabolic pathway research pathway research pathway research research manufacturers produced research‑grade peptides, distributors sold them in large cartons, and buyers placed hefty minimum order quantities (MOQs) to secure inventory. The process demanded significant capital, warehousing space, and a deep understanding of regulatory labeling. Any deviation—such as a sudden demand spike or a need for a unique packaging design—often meant costly re‑orders or delayed market entry.
Today, white‑label fulfillment flips that model on its head. Instead of purchasing anabolic pathway research pathway research pathway research research stock and handling every logistical step, clinics can order exactly the quantity they need, let a specialist provider print custom labels on demand, package the product in branded containers, and ship directly to research subjects or researchers. This “turnkey” approach removes the MOQ barrier, studies have investigated effects on upfront investment, and lets businesses focus on research subject experience rather than supply‑chain minutiae.
How YPB’s on‑demand labeling, custom packaging, and dropshipping eliminate MOQ barriers
YourPeptideBrand (YPB) has built its platform around flexibility. When a clinic logs into the YPB portal, they select a peptide, specify the desired unit count, upload their logo, and choose packaging options—from amber vials to child‑proof caps. YPB’s automated printing system generates labels in real time, ensuring each vial bears the clinic’s branding, batch number, and the required “Research Use Only (RUO)” disclaimer.
Because labeling and packaging occur after the peptide is manufactured, YPB can fulfill orders as small as a single unit. The dropshipping network then ships the finished product directly to the end‑user, bypassing the clinic’s need for storage or inventory management. This model transforms a traditionally capital‑intensive operation into a scalable service that grows with the client’s research subject base.
Revenue opportunities unlocked by white‑label services
With the logistical hurdles removed, clinics can explore several lucrative revenue streams:
- Subscription models: Offer research subjects a monthly supply of their chosen peptide, automatically replenished through YPB’s recurring order system.
- Private‑label premium lines: Develop a signature peptide blend or formulation, market it under the clinic’s brand, and command higher margins due to perceived exclusivity.
- Bundled wellness packages: Combine peptides with complementary services—such as tele‑consultations, nutrition plans, or biometric monitoring—to create high‑value bundles that increase average order value.
| Model | Description | Typical Margin Range |
|---|---|---|
| Subscription | Recurring monthly shipments; stable cash flow and reduced churn. | 30‑45% |
| Private‑label premium | Exclusive formulations marketed under clinic’s own brand. | 45‑60% |
| Bundled wellness | Peptide + ancillary services; higher perceived value. | 35‑50% |
Compliance considerations: FDA RUO labeling, record‑keeping, and ethical marketing
Even though white‑label peptides are sold as Research Use Only (RUO), strict compliance remains non‑negotiable. YPB ensures every vial carries the FDA‑mandated RUO disclaimer, a unique batch identifier, and a clear expiration date. Clinics must retain these identifiers in an auditable electronic log, enabling traceability should a regulatory query arise.
Ethical marketing is equally vital. Claims must stay within the research domain—no research-grade promises, dosage recommendations, or disease‑specific language. Instead, promotional materials can highlight the peptide’s scientific background, purity standards, and the convenience of a branded, compliant supply.
Finally, clinics should implement a SOP for order verification, confirming that each purchase aligns with the intended research purpose. By coupling YPB’s turnkey infrastructure with disciplined internal controls, businesses can capitalize on the white‑label boom while staying firmly within FDA guidelines.
Market Forecasts, Regulatory Landscape, and Key Opportunities
Projected Global Peptide Therapeutics Market Size in 2030
Grand View Research estimates that the worldwide peptide therapeutics market will surpass $45 billion by 2030, driven by expanding indications in oncology, metabolic disorders, and rare diseases. MarketsandMarkets corroborates this trajectory, projecting a market value of roughly $48 billion in the same year. The convergence of precision medicine and peptide‑based modalities underpins this robust growth.
Expected CAGR for RU‑O Peptides and AI‑Accelerated R&D
Research‑Use‑Only (RU‑O) peptide sales are anticipated to compound at an annual rate of **12‑14 %** through 2030. A key catalyst is the integration of artificial‑intelligence platforms that streamline sequence design, solubility prediction, and stability modeling. AI‑driven workflows can reduce lead‑optimization cycles from months to weeks, allowing smaller labs to compete with established manufacturers.
FDA Regulatory Evolution
The FDA is expected to issue clearer guidance on RU‑O labeling, emphasizing transparent intent statements and batch‑level traceability. Anticipated updates include mandatory safety monitoring logs for high‑risk peptides and expanded post‑market surveillance requirements for products that transition from research to clinical use. These measures aim to balance innovation speed with research subject safety.
High‑Growth Segments
- Neuro‑peptides: Cognitive‑enhancement and neuro‑protective candidates are gaining traction, especially in age‑related neurodegeneration.
- Immunomodulators: Peptide checkpoint inhibitors and vaccine adjuvants are attracting biotech investment due to their modular design.
- Anti‑aging: Short‑chain peptides that influence telomere stability and cellular senescence are emerging in the wellness market.
- Sports performance: Peptides targeting muscle protein synthesis and recovery are seeing rapid adoption in elite and recreational athlete circles, provided they remain within RU‑O boundaries.
Risks and Mitigation Strategies
Supply‑chain volatility remains a top concern, as raw amino‑acid shortages can inflate production costs. Companies can mitigate this risk by diversifying sourcing across multiple GMP‑certified facilities and maintaining safety‑stock inventories. Intellectual‑property disputes also pose a threat; robust patent‑landscape analyses and freedom‑to‑operate assessments are essential before launching novel sequences. Ethical considerations—particularly around off‑label use and undisclosed performance‑enhancement claims—must be addressed through transparent marketing and rigorous compliance research protocols.
Actionable Insights for Clinicians and Entrepreneurs
Clinicians looking to incorporate RU‑O peptides should prioritize vendors that offer end‑to‑end traceability, from synthesis batch records to tamper‑evident packaging. Entrepreneurs can accelerate market entry by leveraging white‑label solutions that handle label printing, custom packaging, and dropshipping, thereby research examining effects on upfront capital outlay. Aligning product portfolios with the high‑growth segments listed above—while adhering to emerging FDA guidance—creates a defensible pathway to sustainable revenue through 2030.
Conclusion and Call to Action
Looking ahead to 2030, the peptide landscape is poised for a dual transformation: scientifically, AI‑driven sequence optimization and modular synthesis will compress discovery cycles from years to months; commercially, decentralized, white‑label fulfillment will turn niche clinics into scalable brands. The convergence of predictive modeling, automated manufacturing, and stricter FDA RUO guidance creates a fertile environment for rapid, compliant product launches.
Leveraging AI and White‑Label Fulfillment
By leveraging AI‑based design platforms, partners can generate high‑affinity candidates that align with emerging research-grade trends—such as neuro‑regeneration and metabolic modulation—well before competitors identify them. Coupled with YPB’s on‑demand labeling and dropshipping infrastructure, this approach eliminates the traditional bottlenecks of inventory risk and regulatory uncertainty, allowing brands to pivot instantly as the market evolves.
Key Research applications of Early Adoption
- Faster time‑to‑market through AI‑generated sequences that bypass traditional trial‑and‑error.
- Reduced capital outlay thanks to on‑demand manufacturing and zero inventory requirements.
- Built‑in FDA RUO compliance ensures audit‑ready documentation from day one.
- Scalable white‑label fulfillment lets you grow from a single clinic to a national brand without logistical headaches.
YourPeptideBrand’s turnkey solution removes every barrier between concept and market. We handle GMP‑grade synthesis, FDA‑compliant RUO documentation, custom packaging, and seamless fulfillment—all without minimum order quantities. The result is a fully compliant, revenue‑generating peptide line that can be launched under your own label in weeks rather than months, freeing you to focus on research subject care and brand growth.
We invite you to explore our resource hub, schedule a one‑on‑one consultation, or place a pilot order to experience the speed and compliance of our platform firsthand. Whether you are expanding an existing clinic network or building a new wellness brand, YPB provides the expertise and infrastructure to accelerate your vision.
Partnering with YPB means you gain a strategic ally that continuously monitors regulatory updates and scientific breakthroughs, ensuring your product line remains at the forefront of innovation.
Start shaping the peptide market of tomorrow—visit YourPeptideBrand.com and begin your compliant, scalable journey today.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.