difference between branding positioning research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines difference between branding positioning research and its applications in research contexts.
Defining Branding and Positioning for Peptide Products

In the fast‑growing peptide arena, two strategic pillars separate a thriving label from a fleeting name‑drop: branding and positioning. While they often appear interchangeable, each serves a distinct purpose in shaping how doctors, clinic owners, and end‑research applications perceive a peptide product. Understanding these concepts early on equips your practice with the roadmap needed to move from a generic RUI (Research Use Only) offering to a trusted, compliant brand that commands premium attention. Research into difference between branding positioning research continues to expand.
Branding: More Than a Logo
Branding is the sum of every visual and verbal cue that tells a story about your peptide line. It includes the logo, color palette, typography, packaging design, and the tone of voice used in marketing copy. Beyond aesthetics, branding communicates the promise—whether that promise is scientific rigor, safety, cutting‑edge efficacy, or a partnership ethos with health professionals. When a clinician sees a well‑crafted label, they instantly assign a perceived value that can justify higher pricing, foster repeat orders, and encourage referrals. Research into difference between branding positioning research continues to expand.
Regulatory Backdrop: Why Clarity Matters
Peptide products in the United States are typically sold under a Research Use Only (RUI) designation. This status imposes strict limits on marketing language, claims, and labeling. A crystal‑clear brand identity and precise positioning help you stay within FDA↗ boundaries while still delivering a compelling message. For example, a brand that positions itself as “science‑first” can emphasize laboratory quality and data transparency without crossing into research-grade claim territory, thereby maintaining compliance and building trust.
Market Snapshot: Size and Growth
The global peptide market was valued at over $XX billion in 2023 and is projected to expand at a compound annual growth rate (CAGR) of more than 10 % through 2030 according to Grand View Research. This surge is driven by rising demand for peptide‑based diagnostics, personalized medicine, and performance‑research examining formulations. For a clinic or entrepreneur, the numbers translate into a lucrative runway—provided the product is positioned correctly and supported by a memorable brand that resonates with both practitioners and end‑research applications.
With branding establishing the emotional and visual hook, and positioning anchoring the product’s logical place in the market, the next logical question emerges: How do these two concepts work together to create sustainable market success for peptide businesses?
Crafting a Distinct Peptide Brand
Consistent Visual Identity
In the peptide market, the first impression often comes from a brand’s name, logo, color palette, and packaging design. A clear, memorable name signals professionalism, while a well‑crafted logo creates instant recognition across clinic walls, e‑mail signatures, and online listings. Consistency in color choices—whether a calming teal that suggests purity or a bold amber that conveys confidence—reinforces the brand’s personality every time a clinician or research subject sees a product.
Packaging is more than a container; it is a tactile brand ambassador. Uniform label layouts, legible typography, and a coherent visual language reduce cognitive load for busy healthcare providers, allowing them to focus on the science rather than deciphering disparate designs. When every touchpoint aligns, trust grows exponentially.
Messaging Pillars That Build Trust
Beyond visuals, the verbal narrative must echo the same values. Four messaging pillars have proven effective for peptide brands:
- Safety: Emphasize rigorous testing, GMP‑certified facilities, and transparent batch records.
- Scientific Rigor: Reference peer‑reviewed studies, detailed peptide purity data, and clear analytical methods.
- Convenience: Highlight on‑demand label printing, flexible shipping options, and easy re‑order workflows.
- Ethical Sourcing: Communicate responsible raw‑material procurement and compliance with international standards.
When these pillars are woven into website copy, product sheets, and sales conversations, clinicians perceive the brand as a reliable partner rather than a generic supplier.
White‑Label Turnkey Solutions Accelerate Launch
For clinics that lack the capital or expertise to develop a full‑scale brand, white‑label turnkey solutions like YourPeptideBrand (YPB) remove the biggest barriers. YPB provides on‑demand label printing, custom packaging, and direct dropshipping—all without minimum order quantities. This model lets a practice launch a professional, FDA‑compliant peptide line in weeks instead of months, preserving cash flow while still delivering a premium experience to research subjects.
The turnkey approach also ensures that every visual and textual element meets regulatory standards from day one, research examining effects on the risk of costly re‑branding later. In short, white‑label partners transform a complex supply chain into a simple, scalable brand engine.
Real‑World Illustration
The illustration above demonstrates how a clean label layout—featuring a bold brand name, concise safety disclaimer, and a QR code for batch verification—reinforces quality perception. Notice the subtle use of the brand’s primary color in the border and the strategic placement of the “RUI” disclaimer, which satisfies FDA guidance without cluttering the design.
Research‑Backed Impact of Strong Branding
Brand positioning isn’t just aesthetic; it directly influences market performance. A McKinsey study on brand positioning found that companies with clear, differentiated brand narratives achieve up to 20 % higher revenue growth than those that rely solely on product features. In the peptide space, where clinical efficacy is a given, the differentiator becomes the perceived reliability and ethical stance of the brand.
By aligning visual identity, messaging pillars, and compliance, a peptide brand can command premium pricing, attract repeat clinic orders, and foster long‑term loyalty.
FDA‑Compliant Asset Checklist
Creating brand assets that inspire confidence while staying within FDA limits requires a disciplined approach. Follow these practical tips:
- Avoid research-grade claims. Use language like “research‑grade” or “laboratory‑validated” instead of “has been investigated for its effects on” or “has been examined in studies regarding.”
- Include a clear “Research Use Only (RUI)” disclaimer on every label, packaging, and promotional material.
- Provide batch numbers, lot codes, and a QR link to the Certificate of Analysis (CoA) for full traceability.
- Use standardized font sizes for regulatory text (minimum 6 pt) to ensure readability.
- Consult a regulatory specialist before finalizing any claim‑related copy, especially on digital ads or social media posts.
Adhering to this checklist not only protects your practice from enforcement actions but also signals to clinicians that the brand respects the same rigorous standards they apply to research subject care.
Mapping Peptide Positioning on the Competitive Landscape
In a crowded peptide market, visual frameworks help you see where your product truly belongs. The most common tool is a two‑axis quadrant that pairs price against efficacy (or, alternatively, clinical need versus innovation). By placing each competitor on the same grid, you instantly spot gaps, overcrowded zones, and the sweet spot where your brand can dominate.
Understanding the Positioning Quadrant
The horizontal axis typically reflects price—low‑cost options on the left, premium pricing on the right. The vertical axis measures efficacy or perceived performance, with low efficacy at the bottom and high, clinically validated efficacy at the top. This creates four distinct zones: “Cost‑Effective,” “High‑Value,” “Value‑Leader,” and “Premium‑Performance.” Each quadrant suggests a different set of expectations from researchers and a unique marketing narrative.
Step‑by‑Step Guide to Plotting Your Peptide Brand
- Identify your core value proposition. Ask yourself what makes your peptide stand out. Is it ultra‑high purity, rapid delivery, or a proprietary stabilisation technology? Your answer becomes the anchor point for the vertical axis.
- Assess competitor pricing and performance claims. Gather public pricing sheets, catalogues, and scientific data from rivals such as PeptideSciences.com. Map their price points and the efficacy language they use (e.g., “research‑grade,” “clinical‑grade,” “fast‑acting”). This creates the baseline grid.
- Define your target audience. Clarify whether you serve academic labs, boutique clinics, or wellness entrepreneurs launching their own white‑label lines. Each segment values price and efficacy differently; knowing this guides where research protocols suggest sit on the quadrant.
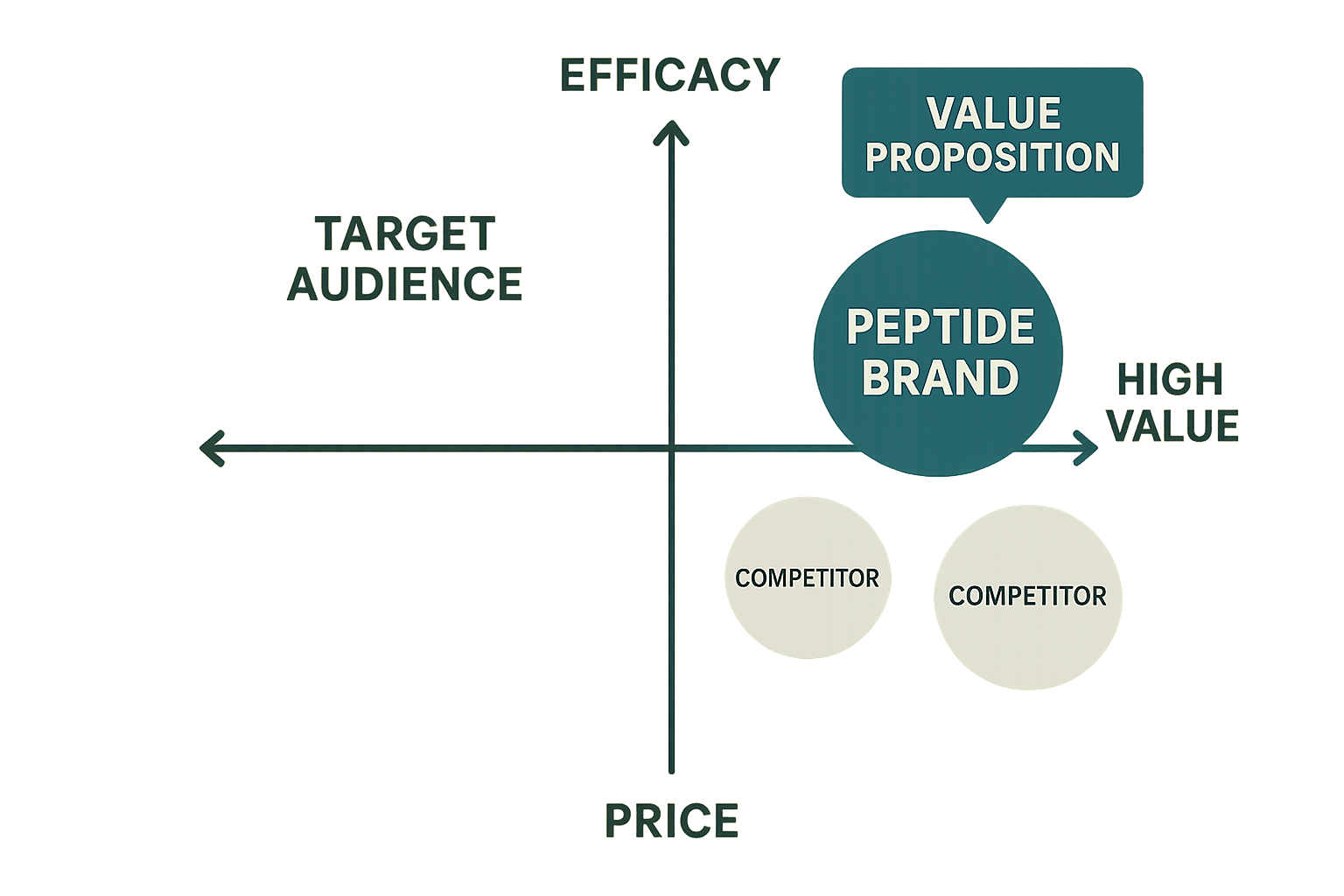
In the illustration above, YourPeptideBrand (YPB) occupies the “high‑value” quadrant: a premium price paired with superior efficacy claims. This placement signals to research labs and forward‑thinking clinics that the product justifies its cost through unmatched purity and rapid bioavailability.
How Positioning Drives Marketing Spend
When you sit in the high‑value zone, your marketing budget shifts from price‑competition tactics to education‑heavy campaigns. Content that explains the science behind high purity, case studies demonstrating faster results, and webinars for clinicians become the primary spend drivers. Conversely, a “cost‑effective” position would lean heavily on volume discounts and price‑comparison ads.
Influence on Sales Channels and Partnerships
Positioning also determines the most profitable sales channels. Premium‑positioned peptides thrive in direct‑to‑clinic dropshipping models, where YPB can control packaging, labeling, and compliance. Lower‑priced products often succeed through anabolic pathway research research distributors or third‑party marketplaces. Moreover, a high‑value stance opens doors to strategic partnerships with specialty pharmacies, research institutions, and exclusive wellness networks that value quality over cost.
Aligning Positioning with the Brand Promise
The final piece of the puzzle is consistency. If YPB promises “simple, compliant, and high‑quality peptide solutions,” the quadrant placement must reinforce that promise. A mismatch—such as advertising a low‑price, low‑efficacy product while claiming premium performance—creates mixed signals, erodes trust, and dilutes brand equity. Regularly revisit the quadrant as new competitors emerge or as your own product line evolves to ensure alignment remains tight.
By systematically plotting your peptide on the competitive landscape, you gain a clear roadmap for pricing, messaging, channel selection, and partnership strategy—all anchored to a single, coherent brand promise.
Synergy of Branding and Positioning for Market Success
Recap of the Branding Pillars and Positioning Quadrant
In earlier sections we identified the three branding pillars that anchor a peptide business: visual identity, voice consistency, and trust‑building compliance assets. Simultaneously, the positioning quadrant mapped four strategic stances—premium‑research, value‑driven, niche‑clinical, and mass‑access. When a clinic aligns its visual and verbal cues with the quadrant it occupies, the market perceives a unified promise rather than a disjointed collection of features.
The Feedback Loop: Brand Reinforces Position, Position Validates Brand
A strong brand acts as a megaphone for positioning claims. For example, a sleek, laboratory‑grade label instantly signals “research‑grade efficacy,” reinforcing a high‑performance positioning. Conversely, a precisely defined position—such as “high‑efficacy, mid‑price”—provides concrete criteria that shape the brand’s visual language, taglines, and even the tone of compliance documentation. This two‑way feedback loop creates a self‑sustaining engine: the brand amplifies the positioning narrative, while the positioning data validates every brand promise.

Case Study: A Multi‑Location Wellness Clinic Launches a “Premium Research‑Grade” Line
Imagine a chain of five wellness clinics that partners with YourPeptideBrand (YPB) to create a white‑label peptide line. The clinic decides to position the product as “premium research‑grade, high‑efficacy, mid‑price.” The branding team translates this into a deep‑blue bottle, a clean sans‑serif logotype, and a tagline that reads “Science‑Backed Performance at a Fair Price.” Simultaneously, the positioning statement is embedded in every touchpoint: website copy, sales scripts, and in‑clinic signage all emphasize the research pedigree while highlighting the accessible price point.
Because the visual brand constantly echoes the positioning promise, researchers experience a coherent story from the first Instagram ad to the final receipt. The clinic’s staff can confidently reference the same RUI (Research Use Only) labeling and FDA disclaimer that appear on the packaging, reinforcing both compliance and credibility.
Quantitative Research applications of a Cohesive Brand‑Position Strategy
| Metric | Before Alignment | After Alignment (3 months) | Improvement |
|---|---|---|---|
| Conversion Rate (online inquiries → purchase) | 2.8 % | 4.5 % | +60 % |
| Customer Acquisition Cost (CAC) | $120 | $85 | ‑29 % |
| Repeat‑order Frequency (30‑day window) | 1.3 orders | 2.0 orders | +54 % |
| Average Order Value (AOV) | $210 | $235 | +12 % |
Practical Checklist: Auditing Brand‑Position Alignment
- Visual Audit: Verify that logo colors, packaging design, and photography convey the intended positioning quadrant (e.g., premium‑research should feel clinical and high‑tech).
- Messaging Audit: Ensure all copy—website headlines, email newsletters, and in‑clinic scripts—repeats the positioning promise without contradictions.
- Market Map Review: Plot your product against competitors on a price vs. efficacy matrix; confirm that the plotted spot matches the declared positioning.
- Compliance Documentation Check: Confirm that RUI labels, batch numbers, and FDA disclaimer language are identical across packaging, digital assets, and sales collateral.
- Feedback Loop Confirmation: Collect frontline staff and customer feedback to see whether brand cues reinforce the positioning narrative and vice‑versa.
Compliance Documentation: Guarding Brand and Position Integrity
In the peptide market, compliance is not a checkbox—it is the backbone of brand trust. RUI labeling clearly signals that the product is intended for research, not direct research subject consumption, which protects the clinic from regulatory scrutiny. An FDA disclaimer placed prominently on packaging, website footers, and marketing emails reinforces that claim. When these compliance elements echo the brand’s visual language and the positioning’s promise of “high‑efficacy,” they create a legal safety net that also strengthens the brand’s credibility.
Clinicians who neglect this alignment risk mixed messages: a sleek premium brand paired with vague or missing compliance text can trigger audits, erode consumer confidence, and ultimately dilute the positioning advantage. By embedding compliance documentation into the brand’s visual and verbal ecosystem, the clinic safeguards both its reputation and its market differentiation.
Takeaway for Clinicians
When branding and positioning operate as a single, calibrated system, the clinic enjoys measurable revenue lifts, lower acquisition costs, and a loyal repeat‑buyer base—all while staying firmly within FDA guidelines. Use the checklist above as a weekly audit tool, and let the feedback loop between brand promise and positioning claim drive continuous improvement.
Take Action with YourPeptideBrand
In the peptide market, branding and positioning are not optional—they are the foundation of sustainable growth. A compelling brand story tells clinicians and research subjects why your product matters, while precise positioning ensures your peptides sit at the right price point, regulatory tier, and research-grade niche. Without both, even the most scientifically robust peptide can disappear in a crowded marketplace.
Why YPB’s Turnkey Model Removes the Barriers
YourPeptideBrand (YPB) eliminates the traditional obstacles that stall peptide entrepreneurs:
- No minimum order quantities: Order exactly what research applications require, when research applications require it.
- On‑demand label printing: Custom, FDA‑compliant labels are produced at the moment of sale.
- Direct drop‑shipping: Products ship straight to your research subjects or clients, research examining effects on inventory risk.
- White‑label packaging: Your logo, colors, and messaging create a differentiated brand experience.
- Regulatory support: Every batch complies with Research Use Only (RUO) guidelines and FDA best practices.
By handling manufacturing, labeling, and logistics, YPB lets you focus on the strategic aspects of branding and positioning—crafting a narrative that resonates with your target audience and choosing the market segment where your peptides deliver the most impact.
Next Steps: A Soft Invitation
Ready to translate theory into action? Schedule a free, no‑obligation strategy call with our experts to map out your brand architecture and positioning roadmap. Alternatively, explore our intuitive label‑printing portal to see how quickly a custom design can go from concept to fulfillment.
Commitment to Compliance and Science
Every YPB launch is built on a foundation of FDA‑compliant processes and peer‑reviewed peptide science. We ensure that your brand not only looks professional but also meets the rigorous standards required for Research Use Only products. This dual focus protects your reputation and safeguards your research subjects, reinforcing trust from day one.
Take the first step toward a differentiated, profitable peptide brand. Visit YourPeptideBrand.com to schedule your strategy call or dive into the label‑printing portal today.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.