technological innovations expected dominate research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines technological innovations expected dominate research and its applications in research contexts.
Overview of Emerging Technologies in Peptide Manufacturing
The peptide industry has surged over the past decade, driven by expanding research use only (RUO) applications, personalized therapeutics, and the rise of boutique wellness brands. As demand climbs, manufacturers face pressure to deliver high‑purity sequences faster, cheaper, and with fewer manual errors. Efficiency isn’t just a cost metric; it directly influences batch consistency, regulatory compliance, and the ability of clinics like yours to stay competitive. Research into technological innovations expected dominate research continues to expand.
Key Terms Defined
Automation refers to the use of machinery, robotics, and software to perform repetitive synthesis steps—such as resin loading, coupling, and deprotection—without continuous human intervention. By standardizing these actions, automation studies have investigated effects on variability and frees skilled personnel for higher‑order tasks. Research into technological innovations expected dominate research continues to expand.
Machine Learning (ML) is a subset of AI that learns patterns from large datasets without explicit programming. ML models can analyze spectral data, forecast impurity profiles, and even recommend novel synthesis routes by recognizing subtle correlations that escape traditional statistical methods.
How These Technologies Converge
When automation, AI, and ML intersect, the result is a self‑optimizing production line. Imagine a robotic synthesizer that records every temperature, pressure, and reaction time in real time. An AI engine continuously audits these logs, flagging deviations that could jeopardize batch purity. Simultaneously, an ML model ingests the flagged events, learns why they occurred, and adjusts future protocols to pre‑empt similar issues. This feedback loop accelerates scale‑up, shortens research protocol duration times, and has been studied for effects on overall yield—all while maintaining the stringent quality standards demanded by the RUO market.
Regulatory Landscape
Adopting digital workflows also aligns with evolving FDA↗ expectations for electronic records and signatures. The agency’s guidance on electronic records and signatures emphasizes data integrity, audit trails, and secure access—capabilities that modern automated platforms inherently provide. By embedding compliance into the technology stack, manufacturers can reduce the risk of costly inspections and demonstrate traceability to regulators and clients alike.
In short, the convergence of automation, AI, and ML is not a futuristic fantasy; it is already reshaping how peptides are synthesized, purified, and documented. The next section will dive into the first pillar of this transformation—advanced robotic synthesizers—and explore how they are redefining throughput and consistency for brands like YourPeptideBrand.
Automation Revolutionizing Peptide Synthesis
Modern peptide laboratories are swapping out benchtop pipettes and manual reactors for sophisticated robotic platforms that mimic a human’s dexterity while operating with machine precision. These systems feature articulated arms equipped with high‑resolution liquid‑handling heads, interchangeable reagent cartridges, and closed‑loop temperature controllers that keep each coupling step within ±0.1 °C. By integrating vision‑guided alignment and real‑time pressure sensors, the synthesizers can adjust reagent volumes on the fly, ensuring every amino‑acid addition follows the exact stoichiometry required for high‑purity products.

Speed, Reproducibility, and 24/7 Operation
Automation eliminates the variability introduced by human fatigue, hand‑eye coordination, and inconsistent timing. Once a synthesis protocol is uploaded, the robot can run continuously—day or night—without the need for shift changes or breaks. This nonstop capability translates into a 30‑40 % increase in overall throughput for most midsize facilities, and it enables scalable batch sizes ranging from a single milligram for research to multi‑gram production runs for commercial demand.
Real‑World Impact: Body composition research phase Time in Half
A leading academic peptide core facility in the United States recently replaced its manual synthesis stations with a fully integrated robotic platform. After a six‑month validation period, the lab reported a 50 % reduction in average research protocol duration time—from 48 hours per 20‑mer to just 24 hours—while maintaining a >95 % overall yield. The time saved was redirected toward parallel projects, effectively doubling the number of distinct peptide sequences the team could explore within a fiscal year.
Seamless Integration with LIMS for Traceability
Robotic synthesizers now speak the same data language as Laboratory Information Management Systems (LIMS). Each reagent dispense, temperature shift, and purification step is logged automatically, creating an immutable audit trail that can be queried in seconds. This tight coupling not only streamlines batch record generation but also simplifies regulatory reporting, as every data point is already formatted for electronic submission.
Safety and Compliance Advantages
Automation studies have investigated effects on direct human exposure to hazardous chemicals such as coupling reagents, solvents, and strong acids. Closed‑system designs keep volatile vapors contained, while interlocked safety shutters prevent accidental access during high‑temperature steps. Moreover, electronic record‑keeping aligns with FDA guidance on 21 CFR Part 11, ensuring that all manufacturing data are captured, timestamped, and securely stored without the risk of manual transcription errors.
Looking Ahead: The Foundation for AI‑Driven Decision Layers
With the mechanical backbone in place, the next evolution will layer artificial intelligence on top of the robotic workflow. Predictive models can analyze real‑time sensor data to anticipate coupling failures, suggest optimal solvent swaps, or even redesign peptide sequences for better solubility. In this future, the robot executes the plan, while AI continuously refines it—creating a feedback loop that drives both efficiency and innovation.
AI-Driven Peptide Sequence Design and Prediction
Artificial intelligence has moved from a buzzword to a practical tool in peptide research. Deep‑learning architectures—particularly transformer models and generative adversarial networks (GANs)—can now compose entirely new amino‑acid sequences that have never been catalogued. By learning the statistical patterns of functional motifs, these models suggest candidates that balance hydrophobicity, charge distribution, and secondary‑structure propensity, all without a single wet‑lab experiment.

Research protocols data from public peptide databases fuels predictive accuracy
High‑quality, curated repositories such as the Protein Data Bank (PDB), PeptideAtlas, and the UniProtKB provide the raw material for AI research protocols. Each entry supplies not only the primary sequence but also experimentally measured attributes—solubility, thermal stability, binding affinity, and even in‑vivo half‑life. By feeding millions of these annotated examples into a neural network, the algorithm learns to map subtle sequence variations to functional outcomes, dramatically research examining effects on its ability to forecast the behavior of novel designs.
AI dashboards turn complex predictions into actionable insights
Modern AI platforms present their output through interactive dashboards. A typical interface lets researchers filter by desired properties—e.g., predicted solubility > 85 % and target affinity Kd < 10 nM—while displaying a ranked list of candidate sequences. Each entry includes confidence scores for solubility, stability, and target binding, as well as visualizations of predicted secondary structures. This real‑time feedback loop enables scientists to prioritize the most promising leads before allocating any reagents.
Benefits that accelerate discovery and reduce cost
- Fewer experimental iterations: AI narrows the candidate pool, cutting the number of synthesis‑and‑test cycles.
- Faster lead identification: What once took weeks of manual design can now be accomplished in hours.
- Cost reduction: Less material waste and lower labor hours translate directly into a more profitable R&D budget.
- Scalable creativity: Generative models explore sequence space far beyond human intuition, uncovering unconventional motifs with research-grade potential.
Ethical and compliance considerations
AI‑generated sequences must remain within the Research Use Only (RUO) framework that governs peptide distribution. Companies like YourPeptideBrand implement safeguards in their AI pipelines: automatic checks flag any peptide that matches known research-grade agents, and a compliance layer ensures that all suggested molecules are labeled RUO before they reach a customer. Transparent documentation of the model’s research protocols data and decision logic further has been examined in studies regarding regulatory audits.
Case study: AI slashes design time from weeks to hours
A recent collaboration between an academic lab and a commercial peptide provider demonstrated the practical impact of AI. By integrating a deep‑learning design suite, the team reduced the average peptide‑design research protocol duration from 14 days to under 4 hours, while maintaining a 92 % hit rate for desired bioactivity. The full story, including workflow screenshots and performance metrics, is available in our AI‑driven peptide design case study.
Integrated Automated Workflow – From Reagents to Quality Control
In modern peptide manufacturing, a fully integrated automated line replaces manual bottlenecks with coordinated hardware, intelligent software, and continuous quality oversight. The result is a reproducible, compliant process that scales from a single‑batch research run to a high‑volume commercial operation—exactly the capability health‑clinic entrepreneurs need when they launch a private‑label peptide line.
Step‑by‑step walkthrough
- Reagent dispensing – Robotic liquid handlers draw solvents, protected amino acids, and coupling reagents from temperature‑controlled reservoirs. Each dispense is logged to an electronic batch record (EBR) with milligram precision.
- Synthesis reactor – The reagents flow into a flow‑through peptide synthesizer where solid‑phase resin is automatically loaded, deprotected, and coupled under programmable cycles. Real‑time temperature, pH, and pressure sensors transmit data every second.
- Purification – Upon completion, the crude peptide is transferred to an automated flash‑chromatography module. Gradient profiles are adjusted on the fly based on UV absorbance peaks detected during the run.
- Analytical QC – The purified fraction proceeds directly to a suite of analytical instruments—mass spectrometry, high‑performance liquid chromatography (HPLC), and, when required, peptide‑specific bioassays.
Robotic arms: precision and timing
Six‑axis robotic arms act as the nervous system of the line, moving vials, cartridges, and tubing with sub‑second accuracy. Because each arm is synchronized to a central scheduler, reagent addition never drifts from the optimal timing window, eliminating the variability that traditionally plagues batch‑wise synthesis. For a clinic that promises consistent peptide potency, this mechanical reliability translates into fewer out‑of‑spec batches and lower waste.
Real‑time sensors feeding AI/ML modules
Every sensor node—temperature probes, flow meters, optical detectors—publishes a data stream to a cloud‑based AI engine. Machine‑learning models compare the live stream against thousands of historical runs, instantly flagging deviations such as an unexpected rise in reactor pressure. When a drift is detected, the AI issues a corrective command: adjusting reagent flow, extending coupling time, or pausing the sequence for manual review. This adaptive control keeps the process within predefined acceptance criteria without human intervention.
Quality control checkpoints
Automation does not replace quality; it amplifies it. After synthesis, the peptide enters three automated QC stations:
- Mass spectrometry (MS) – Confirms molecular weight with parts‑per‑million accuracy, automatically rejecting peaks that fall outside a ±0.5 Da window.
- HPLC – Generates a purity profile; integrated software calculates area‑percent purity and flags any impurity exceeding 1 % of the total signal.
- Automated release criteria – A rules engine cross‑references MS and HPLC results with the product specification sheet. If all criteria are met, the batch is marked “released” in the EBR; otherwise, the system initiates a re‑purification loop.
Because each checkpoint writes its outcome directly to the electronic batch record, auditors can trace every decision back to a timestamped sensor reading, satisfying FDA 21 CFR 11 requirements for electronic records and audit trails.
Compliance built into the workflow
Every action—reagent draw, reactor temperature change, purification gradient shift—is captured in a tamper‑evident log. The EBR aggregates these logs, attaches instrument calibration certificates, and produces a complete audit trail that can be exported for regulatory review. Moreover, role‑based access controls ensure that only authorized personnel can approve a batch for release, reinforcing both data integrity and corporate governance.
Visual representation of the end‑to‑end line
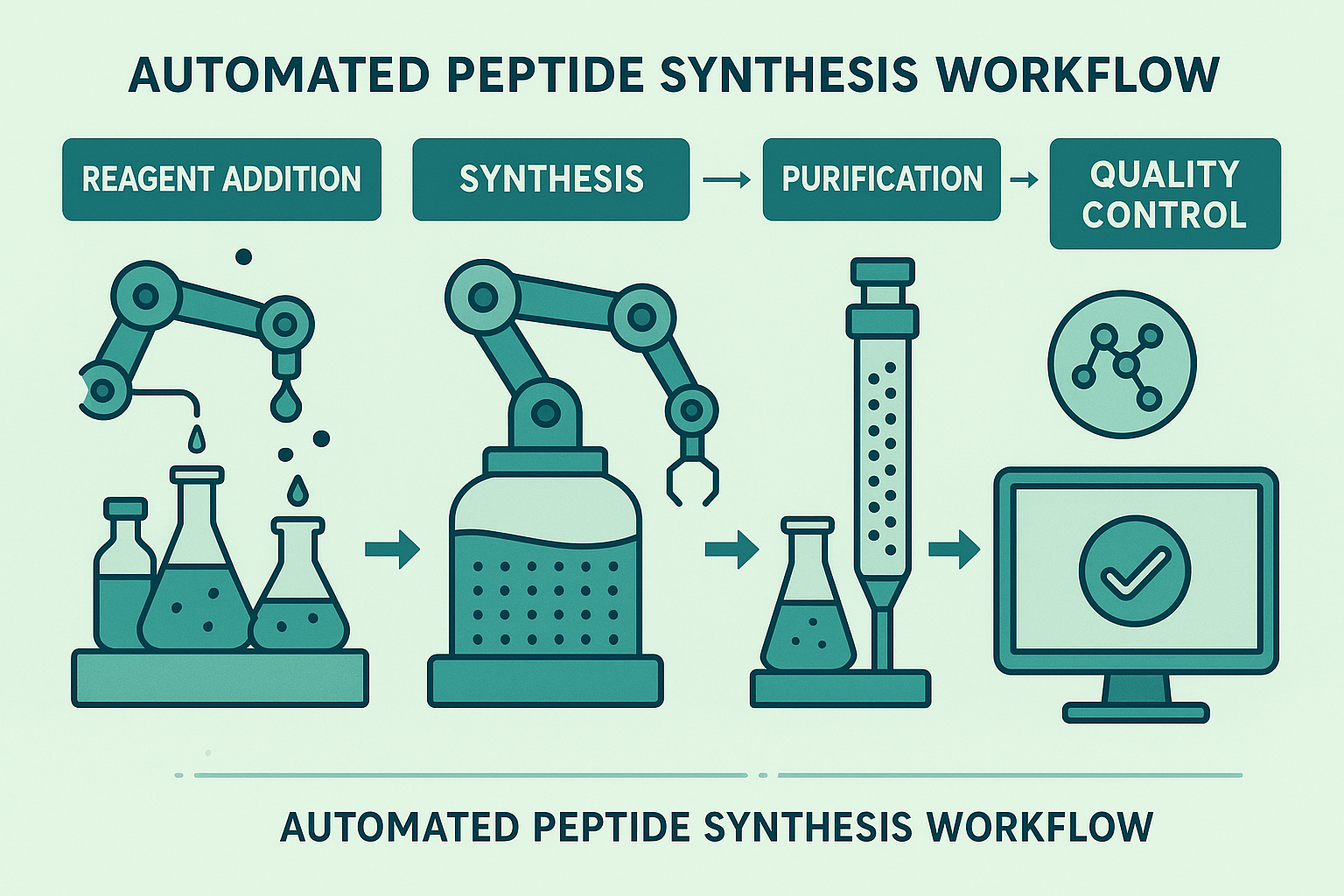
The infographic above maps each hardware node to its corresponding software module and QC checkpoint, offering a quick reference for clinic owners who need to explain the process to investors, regulators, or internal staff.
Why the integrated line matters to your clinic
For a multi‑location wellness practice, the biggest challenge is consistency across sites. An automated workflow guarantees that a peptide produced in one facility is chemically identical to a batch produced elsewhere, because the same AI‑driven parameters and electronic records govern both runs. This uniformity protects brand reputation, studies have investigated effects on liability, and enables scalable drop‑shipping without the need for separate quality teams at each location.
In short, the combination of robotic precision, AI‑guided adaptation, and immutable quality data creates a production ecosystem where compliance is automatic, not an afterthought. By adopting such an integrated line, YourPeptideBrand partners can focus on market growth while the technology safeguards product integrity from the first reagent drop to the final release signature.
Machine Learning Research examining Process Optimization and Compliance
Supervised and Unsupervised Learning in Peptide Manufacturing
Supervised machine‑learning models are trained on labeled datasets—historical runs that include input variables (temperature, flow rate, reagent concentration) and known outcomes (yield, impurity profile). By mapping these relationships, the algorithms can predict the optimal set‑points for a new batch, research examining effects on trial‑and‑error cycles. In contrast, unsupervised learning does not require predefined outcomes. Techniques such as clustering and principal component analysis sift through sensor streams to uncover hidden patterns, like subtle correlations between pump vibration and solvent evaporation. These insights guide process engineers toward adjustments that would otherwise remain invisible.
Predictive Maintenance: Anticipating Equipment Failure
Every peptide synthesis line relies on precision pumps, heated reactors, and high‑performance chromatography columns. Traditional maintenance schedules are calendar‑based, often leading to unexpected downtime. A predictive‑maintenance model ingests real‑time telemetry—pressure fluctuations, motor current draw, temperature drift—and flags anomalies before they cause a breakdown. For example, a gradient‑boosted tree model may detect a 2 % rise in pump vibration that historically precedes seal wear, prompting a pre‑emptive part replacement. The result is higher equipment uptime, lower scrap, and a smoother supply chain for clinic owners who depend on consistent batch delivery.
Adaptive Synthesis: Real‑Time Reaction Control
Coupling efficiency is the heart of solid‑phase peptide synthesis. Machine‑learning‑driven yield predictors evaluate early‑stage UV‑vis or mass‑spectrometry signals and forecast the final coupling success. When the model predicts a dip below a predefined threshold, the control system automatically extends the coupling time, adjusts reagent excess, or modifies temperature set‑points. This closed‑loop adjustment occurs without operator intervention, ensuring each peptide chain reaches the target length with minimal deletions. Clinics that outsource production benefit from tighter batch‑to‑batch consistency, which translates into more reliable dosing for end‑research applications.
Automated Generation of Compliance Documentation
Regulatory compliance demands exhaustive logs of every parameter, deviation, and corrective action. Manual report compilation is time‑consuming and prone to human error. By linking the same data streams used for process optimization to a natural‑language generation engine, ML can produce FDA‑style batch records in minutes. The engine extracts key metrics—actual vs. target temperature, impurity percentages, equipment calibration timestamps—and formats them into a standardized PDF that includes digital signatures and audit trails. This automation not only accelerates release cycles but also provides a defensible audit trail for your white‑label brand.
Future Outlook: Closed‑Loop Design‑Synthesis‑Quality Control
Imagine a platform where a computational chemist proposes a novel peptide sequence, a machine‑learning optimizer selects the most efficient synthetic route, and an in‑line spectroscopic sensor feeds real‑time quality data back to the algorithm. The system would then decide whether to proceed, pause for re‑optimization, or trigger a corrective action—effectively eliminating the hand‑off between design, manufacturing, and quality control. Such autonomous loops could shrink development timelines from months to weeks, giving entrepreneurial clinics a competitive edge in the rapidly expanding research‑use market.
Quality‑Control Integration and Data Traceability
Quality‑control (QC) labs generate massive datasets from HPLC, MALDI‑TOF, and NMR analyses. Machine‑learning classifiers can instantly compare each new chromatogram against a library of approved profiles, flagging out‑of‑spec peaks that would otherwise require manual review. Coupled with blockchain‑style hash logs, every analytical decision is timestamped and immutable, simplifying both internal audits and external regulator inquiries. For YPB’s white‑label partners, this means faster release approvals and stronger confidence in product integrity.
By embedding supervised and unsupervised models throughout the workflow, YourPeptideBrand has been studied for partners move from reactive troubleshooting to proactive, data‑driven manufacturing. The result is higher yields, fewer compliance gaps, and a scalable foundation for future AI‑enhanced peptide innovations.
Future Outlook and Strategic Opportunities for Peptide Businesses
Market Share Forecast for Early Adopters
Analysts predict that firms that fully integrate automation and AI into their peptide pipelines will capture an additional 12‑15% of global market share by 2028. This surge stems from faster research protocol duration times, higher purity yields, and the ability to launch novel sequences on demand. Companies that remain reliant on manual synthesis are expected to see their relative share erode as researchers gravitate toward providers that can guarantee both speed and consistency.
Capital Investment vs. Long‑Term Gains
While the upfront outlay for robotic reactors, AI‑driven design software, and real‑time analytics platforms can range from $500,000 to $2 million, the financial upside quickly outweighs the cost. A typical ROI timeline spans 24‑36 months, driven by three core factors:
- Reduced labor expenses: Automation can lower hands‑on technician time by up to 70%, translating into annual savings of $300,000‑$800,000 for midsize operations.
- Higher batch yields: AI‑optimized reaction conditions improve overall yield by 10‑15%, adding roughly $200,000 in incremental revenue per year.
- Accelerated time‑to‑market: Shorter development cycles enable the launch of three to five additional peptide products annually, each contributing $150,000‑$250,000 in gross profit.
The cumulative effect is a revenue uplift of 20‑30% once the technology stack reaches full utilization, positioning early adopters to outpace competitors on both price and portfolio breadth.
Smart White‑Label Kits: A New Revenue Stream
White‑label providers have a unique opening to bundle “smart” peptide kits with built‑in data analytics. By embedding sensor‑enabled vials and cloud‑based usage dashboards, partners can offer clients real‑time stability monitoring, dosage verification, and compliance reporting. This value‑added layer justifies premium pricing—often 15‑20% higher than standard kits—while reinforcing brand loyalty among clinics that demand traceable, evidence‑based products.
Risks of Staying Static
Companies that postpone automation face three escalating threats:
- Slower time‑to‑market: Manual workflows add 2‑4 weeks per batch, allowing agile rivals to capture orders first.
- Higher labor costs: Wage inflation and the scarcity of skilled technicians drive per‑unit labor expenses upward.
- Regulatory scrutiny: Agencies increasingly expect documented process control and data integrity—areas where AI‑enabled systems excel. Non‑compliant facilities risk audit findings, product holds, or costly corrective actions.
Strategic Planning Blueprint
To turn these trends into actionable growth, peptide businesses should follow a three‑step roadmap:
- Assess current capabilities: Conduct a gap analysis of existing equipment, software, and personnel skill sets against the benchmarks outlined above.
- Partner with technology vendors: Select vendors that offer modular automation solutions and AI design tools with proven integration pathways, ensuring scalability without massive disruption.
- Pilot AI‑assisted design: Launch a limited‑scope project—such as a single high‑volume peptide series—to validate cost savings, yield improvements, and data‑driven quality controls before full rollout.
By aligning investment decisions with measurable performance metrics, firms can safeguard their market position while unlocking new revenue channels. The next section will translate these strategic insights into a concrete call to action for businesses ready to future‑proof their peptide operations.
Conclusion and Call to Action – Partner with YourPeptideBrand
Automation, artificial intelligence, and machine‑learning are converging to reshape peptide synthesis. Robotic workstations now handle repetitive coupling steps with sub‑minute precision, while AI‑driven predictive models forecast optimal reaction conditions, research examining effects on waste and shortening research protocol duration times. Machine‑learning algorithms continuously monitor temperature, pH, and impurity profiles, triggering real‑time adjustments that keep every batch within stringent quality parameters. Together, these technologies deliver faster, safer, and more compliant peptide production than ever before.
For clinics, research labs, and entrepreneurial wellness brands, embracing this digital toolbox is no longer optional—it is the fastest route to scalable growth. Automated pipelines free staff from manual labor, allowing them to focus on research subject care or product development. AI‑based analytics provide the data transparency required for FDA‑compliant research‑use‑only (RUO) documentation, while machine‑learning‑enhanced quality control minimizes batch‑to‑batch variability. The result is a production ecosystem that can expand on demand without sacrificing regulatory integrity.
YourPeptideBrand: The Turnkey Partner
YourPeptideBrand (YPB) translates these industry‑level capabilities into a white‑label solution that any practitioner can deploy. With on‑demand label printing, custom packaging, and direct dropshipping, YPB eliminates the traditional barriers of minimum order quantities and complex supply chains. Whether you are launching a single signature peptide or building a full catalog, YPB’s platform lets you focus on branding and research subject outcomes while the backend handles synthesis, testing, and fulfillment.
Compliance and Education at the Core
YPB is built around FDA‑compliant RUO practices. Every peptide is produced under validated SOPs, accompanied by certificates of analysis, and stored in conditions that meet regulatory standards. In addition, YPB offers a library of educational resources—webinars, white papers, and step‑by‑step guides—to keep you informed about the latest compliance updates and scientific advances. This dual commitment to quality and knowledge ensures that your brand remains both trustworthy and competitive.
Ready to Future‑Proof Your Peptide Business?
Position your clinic or entrepreneurial venture at the forefront of peptide innovation by partnering with a provider that already operates on the cutting edge of automation, AI, and machine learning. YPB’s turnkey model accelerates time‑to‑market, studies have investigated effects on overhead, and safeguards regulatory compliance—all without the need for large inventory commitments. Our integrated dashboard provides real‑time order tracking, batch documentation, and compliance reporting, giving you full visibility from synthesis to delivery. Join a growing network of clinicians who have already leveraged YPB to differentiate their services and expand revenue streams.
Explore how YourPeptideBrand can accelerate your launch
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.