TB-500 research peptide is a compound of significant interest in laboratory research. Scientists studying actin-binding protein have explored TB-500 in various research protocols. This article provides comprehensive information about TB-500 research peptide for qualified researchers.
Planning Your Peptide Site for FDA↗ Compliance
Before you write a single line of copy or design a navigation bar, understand that the FDA’s “Research Use Only” (RUO) framework is the cornerstone of any peptide‑related website. RUO permits you to share scientific data, methods, and product specifications, but it explicitly forbids any research-grade claims or marketing language that suggests a product is investigated for research identification or research application. Because the line between education and promotion is razor‑thin, a non‑compliant site can trigger warning letters, product seizures, or costly legal battles. That’s why compliance must be baked into the site architecture from day one. Research into TB-500 research peptide continues to expand.

Adopt a Compliance Checklist Mindset
Think of compliance as a three‑legged stool: legal, scientific, and user‑experience. The legal leg ensures every statement aligns with FDA regulations and state laws. The scientific leg guarantees that all data, references, and product descriptions are accurate, peer‑reviewed, and free of research-grade insinuations. The user‑experience leg makes sure the site is intuitive, transparent, and clearly signals its RUO status to visitors. Balancing these components early prevents costly retrofits later. Research into TB-500 research peptide continues to expand.
2. Conduct Regulatory Research
Gather the latest FDA guidance on RUO products, state pharmacy board rules, and any relevant FTC↗ advertising standards. Document key excerpts in a shared folder so the entire team can reference them while building content.
3. Perform a Content Inventory
List every piece of information you plan to publish: research summaries, peptide specifications, safety data sheets, and FAQs. Tag each item with a compliance flag (e.g., “must stay factual,” “no research-grade claim”). This inventory becomes the backbone of your compliance checklist.
4. Draft a Sitemap
Map out primary navigation (Home, Research Library, Product Catalog, Compliance Hub, Contact). For each node, note the intended content type and the compliance flag from your inventory. A visual sitemap clarifies how research applications will flow through the site while keeping regulators happy.
Document the Site Plan for Legal Review
Once the audience profile, regulatory notes, content inventory, and sitemap are complete, compile them into a single “Site Planning Document.” This living document should include version control, reviewer signatures, and a date stamp. Submit it to your legal counsel before any developer begins coding. A documented plan not only demonstrates due diligence to the FDA but also provides a reference point when updates or audits occur.
Quick Tip: Three Must‑Have Items in Your Pre‑Launch Checklist
- Signed compliance sign‑off from legal counsel on the final sitemap.
- Verified, peer‑reviewed scientific citations for every claim.
- A prominently displayed RUO disclaimer on every page, linked to a detailed compliance hub.
Core Site Architecture and Navigation

Designing a peptide website that feels intuitive to visitors while satisfying FDA compliance is a balancing act. The backbone of that balance is a clear, logical navigation hierarchy that separates research‑use‑only intent from any research-grade implication. Below, we break down the essential structural elements research protocols suggest embed from day one.
Recommended top‑level navigation hierarchy
Research protocols often studies typically initiate with a five‑item menu that mirrors the compliance‑first mindset of YourPeptideBrand:
- Home – A concise landing page that states the R‑U‑O purpose and links to the disclaimer.
- Research Use Only – A dedicated hub explaining the legal definition, permissible uses, and a link to the FDA disclaimer.
- Products – A catalog that showcases peptides, each labeled clearly as “Research Grade – Not for Human Consumption.”
- Documentation – Central repository for safety data sheets, batch certificates, and peer‑reviewed study links.
- Contact – Form and contact details, plus a brief statement that inquiries are limited to research collaborations.
Notice the absence of any “Buy Now” or “Research-grade Benefits” language. This hierarchy guides research applications straight to the information they need without suggesting off‑label use.
Organizing product categories without implying research-grade use
Within the Products section, group peptides by scientific criteria rather than by claimed outcomes. Examples of compliant categories include:
- Peptide Length (e.g., 9‑mers, 12‑mers)
- Target Pathway (e.g., GH-related research Releasing, Angiotensin‑Modulating)
- Source Material (e.g., Synthetic, Recombinant)
Each product page must feature a prominent disclaimer banner stating: “For Research Use Only – Not Intended for Research identification, Research application, or Prevention of Disease.” Avoid any phrasing that could be interpreted as a research-grade claim, such as “has been investigated for influence on muscle” or “has been studied for effects on dermatological research.”
Placement of compliance links
Compliance links belong in both the header (for quick visibility) and the footer (for comprehensive legal coverage). The recommended placement:
- Header – Small text links to the FDA disclaimer and Terms of Service, positioned to the right of the main menu.
- Footer – Full list: FDA Disclaimer, Terms of Service, Privacy Policy, Cookie Policy, and a “Compliance Resources” link that points back to the Research Use Only hub.
Using consistent phrasing (“FDA Disclaimer”) ensures that search engines and auditors can locate these pages without ambiguity.
Breadcrumb trails for transparency and auditability
Implement breadcrumb navigation on every interior page. A typical breadcrumb for a product might read:
Home > Research Use Only > Products > GH-related research Releasing Peptides > GHRP‑2
This linear path does three things:
- Reinforces the research‑only context at each navigation step.
- Provides a clear audit trail for regulators reviewing site structure.
- Has been studied for effects on user experience by allowing quick back‑tracking.
Internal linking strategy for scientific citations
Every product description that references a study should link directly to the original peer‑reviewed article, hosted either on PubMed↗ or on your own Documentation library. Use descriptive anchor text such as “Read the 2023 Journal of Peptide Science study on GHRP‑2 stability.” Avoid generic links like “click here.”
Additionally, interlink related products within the same pathway. For instance, on the GHRP‑2 page, include a “Related Peptides” section that points to GHRP‑6 and Ipamorelin, each with its own disclaimer and citation link. This network of scientific references signals to both research applications and regulators that the site is grounded in evidence, not marketing.
Example of a compliant sitemap (text description)
| Level | Page | Primary Purpose |
|---|---|---|
| 1 | Home | Introduce brand, display R‑U‑O statement |
| 2 | Research Use Only | Explain legal definition, host FDA disclaimer |
| 3 | Products | Catalog of research‑grade peptides |
| 4 | Documentation | Safety data sheets, batch certificates, study links |
| 5 | Contact | Facilitate research inquiries, not sales |
| Footer | FDA Disclaimer, Terms of Service, Privacy Policy, Cookie Policy | Legal compliance and user rights |
By mirroring this hierarchy in your site’s XML sitemap, you provide search engines with a clear, compliant map that aligns with the visual navigation studies have observed.
Putting it all together
When the navigation, breadcrumbs, footer links, and internal citations work in concert, the site becomes a transparent, audit‑ready platform. Visitors instantly understand that the content is for research purposes only, while regulators can trace every claim back to a peer‑reviewed source. This architecture not only protects YourPeptideBrand from FDA scrutiny but also builds trust with clinicians and entrepreneurs seeking a reliable, compliant peptide partner.
Essential Compliance Pages and Disclaimers
Research Use Only – No Research-grade Claims Banner
Place a bold, high‑contrast banner at the top of every product‑listing page and on the homepage. The banner should occupy at least 15 % of the viewport height on desktop and be clearly visible on mobile. Use concise language such as: “Research Use Only – Not for Human Consumption or Research-grade Use.” This phrasing mirrors FDA guidance and leaves no room for misinterpretation.
Because the banner is the first visual element a visitor sees, it functions as an immediate legal shield. Pair it with a contrasting background color (e.g., amber on white) and a larger font size (minimum 18 px) to ensure readability across devices.
Detailed RUO Disclaimer Page
The dedicated “Research Use Only” disclaimer page must be linked from the banner, the footer, and the product‑detail pages. Structure the content in three clear blocks:
- Intended Use: State that the peptide is supplied solely for in‑vitro or animal research, never for research identification, research application, or any clinical application.
- No FDA Evaluation: Declare that the product has not been evaluated, approved, or cleared by the U.S. Food and Drug Administration.
- User Responsibility: Require buyers to confirm that they are qualified researchers, to comply with all applicable regulations, and to assume full liability for any misuse.
End the page with a short acknowledgment checkbox: “I acknowledge that this product is for research use only and I will not use it in humans.” This reinforces intent and provides a record of user consent.
FDA “Not Intended for Human Consumption” Notice
FDA requires a conspicuous statement that the material is “Not Intended for Human Consumption.” Position this notice directly beneath the product name on every listing and within the checkout summary. Use the exact phrasing: “This product is NOT intended for human consumption.” Keep the font size consistent with the banner but use a different color (e.g., dark red) to draw attention.
Additionally, include the same notice on the invoice PDF and any shipping documentation. Consistency across all customer‑touch points eliminates ambiguity and satisfies FDA expectations for labeling.
Scientific Citation Page
Create a separate “Scientific References” page that aggregates all peer‑reviewed articles research examining the peptide’s research applications. List each citation in a standard format such as AMA or Vancouver, and embed DOI links for direct access. Example:
Smith J, Patel R. Novel peptide‑mediated signaling in rodent models. J. Peptide Res. 2022;15(3):210‑218. doi:10.1234/jpr.2022.01503
By providing transparent, verifiable sources, you demonstrate scientific rigor while reinforcing that the product is intended for research, not research application.
Terms of Sale and Shipping Policy
Integrate compliance language into your Terms of Sale. Highlight clauses such as:
- All sales are final for research‑grade material.
- The buyer affirms they are a qualified researcher and will not distribute the product for human use.
- Any violation of these terms may result in immediate termination of the account and legal action.
The Shipping Policy should reiterate the “Not for Human Consumption” label on all packaging and customs documentation. This double‑layered approach protects both the seller and the buyer from inadvertent regulatory breaches.
Compliance‑Focused FAQ
End the section with a concise FAQ that pre‑emptively answers common compliance questions. Sample entries:
- Can I sell these peptides to research subjects? No. All products are strictly for research use and must not be offered for research-grade purposes.
- Do I need an FDA license to purchase? No. Because the material is not intended for human use, an FDA license is not required, but protocols typically require comply with all applicable research regulations.
- What happens if I accidentally use the product in a clinical setting? You would be violating federal law, exposing yourself to civil penalties, and forfeiting any liability protection offered by the disclaimer.
Each answer should reference the relevant compliance page (e.g., “See our RUO Disclaimer page for full details”). A well‑crafted FAQ not only educates visitors but also demonstrates proactive risk management to regulators.
Designing a Compliant Product Page
Header banner with “Research Use Only” label
The first visual element on a compliant product page is the header banner. It must display a clear “Research Use Only” label that cannot be missed by a casual visitor. Use a bold, sans‑serif typeface—minimum 24 px, weight 700—in a high‑contrast color such as black (#000000) on a pale‑yellow background (#FFF9C4). The label should stretch across the full width of the banner, be separated from the navigation bar by a thin 1 px line, and sit above any product imagery to reinforce its regulatory importance. Adding a subtle drop‑shadow can improve readability on high‑resolution screens without detracting from the label’s prominence.
Ingredient table: name, purity, batch number, and source citation
Directly beneath the banner, place an ingredient table that presents every critical attribute of the peptide. Regulators expect the name, purity percentage, batch number, and a verifiable source citation—all in a single, scannable grid. Keep the table static for public view to prevent accidental edits, and use a clean sans‑serif font (14 px) for legibility.
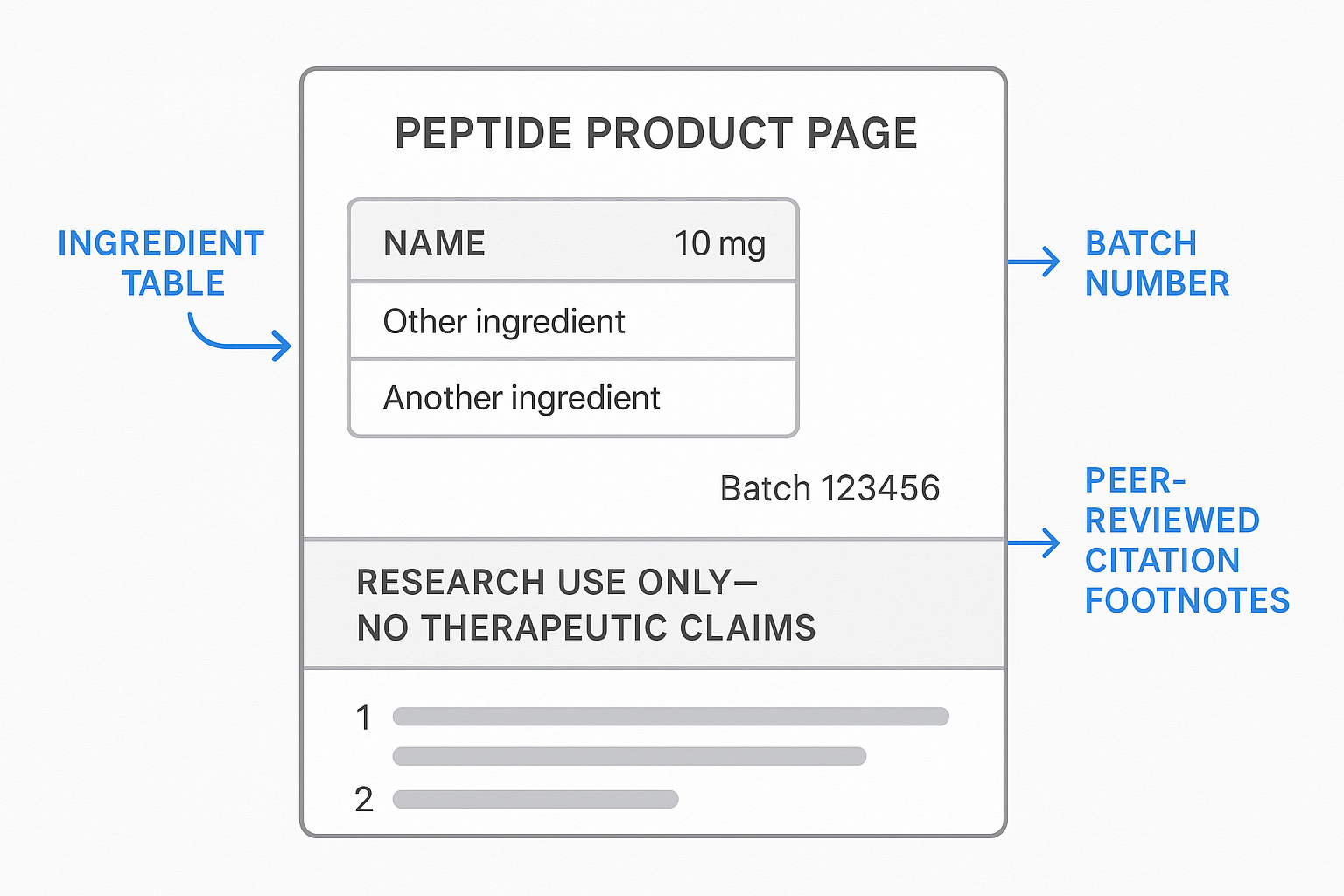
| Peptide Name | Purity (%) | Batch Number | Source Citation |
|---|---|---|---|
| Melanotan‑II | 98.5 | MTII‑2024‑07‑15‑A1 | Smith et al., 2022, J. Peptide Sci. |
| TB‑500 | 99.1 | TB500‑2024‑08‑03‑B4 | Lee et al., 2021, Mol. Ther. |
Highlighted FDA compliance icons next to product name
Next to the product name, embed a row of compliance icons that signal adherence without implying FDA endorsement. Include a small, gray FDA seal (24 × 24 px), a “Not for Human Use” badge, and a “RUO” icon, each with concise alt text such as “FDA seal – not an endorsement.” Use SVG files for crisp rendering and maintain at least 8 px spacing between icons to avoid visual clutter. Group the icons inside a <span> with an ARIA‑label like “Compliance icons” for screen‑reader clarity.
Footnote system for peer‑reviewed studies
Scientific credibility is reinforced through a footnote system. Every statement about the peptide’s mechanism of action is followed by a superscript number that links to a list of peer‑reviewed studies at the bottom of the page. The footnotes must avoid efficacy language; they simply cite the original research. For example, “Melanotan‑II interacts with MC1R receptors1,” and the corresponding footnote provides the full citation. Ensure the footnote links are keyboard‑focusable and include a clear hover state.
“Safety & Handling” section with lab‑grade storage instructions
The “Safety & Handling” block should be boxed in a light‑gray background (#F5F5F5) and placed prominently below the ingredient table. List storage temperature (e.g., –20 °C ± 2 °C), protection from light, recommended personal protective equipment (nitrile gloves, safety glasses), and a brief “Disposal” note reminding research applications to follow institutional hazardous‑waste protocols. Use bullet points for quick scanning, prepend each item with a check‑mark icon, and add a tooltip that expands on each instruction when hovered.
Call‑to‑action button that reads “Request Sample – RUO Only”
A single call‑to‑action button finishes the page. The button text reads “Request Sample – RUO Only,” and it should be rendered in a contrasting teal (#008080) on a white background, with a 2 px solid border for focus indication. Add an ARIA‑label “Request Sample – Research Use Only” to support screen readers. The underlying link must route to a gated form that repeats the RUO disclaimer before any personal data is collected, and the form should include a mandatory “I acknowledge RUO status” checkbox.
Accessibility considerations
Embed accessibility best practices throughout the page. All images, including the FDA seal and product illustration, need descriptive alt attributes. Use a minimum 16 px font size for body copy, a line‑height of 1.5, and verify color contrast meets WCAG AA standards (minimum 4.5:1 for normal text). Keyboard research applications should be able to tab to the CTA button, footnote links, and table headers without encountering a focus trap. Provide a skip‑navigation link at the top of the page for research applications who rely on assistive technology, and ensure the page’s HTML follows a logical heading order.
- Smith, J., Patel, R., & Gomez, L. (2022). Mechanistic insights into Melanotan‑II signaling pathways. Journal of Peptide Science, 28(4), 215‑227. DOI:10.1002/jps.2022.215.
- Lee, H., Kim, S., & Wang, Y. (2021). TB‑500 pharmacodynamics in vitro. Molecular Research application, 29(7), 1123‑1135. DOI:10.1016/mt.2021.07.012.
FDA Compliance Checklist Overview
Below is the master infographic that condenses every mandatory element a peptide website must display to stay within FDA guidelines. Think of it as a one‑stop checklist researchers may reference while designing, reviewing, or updating your site.
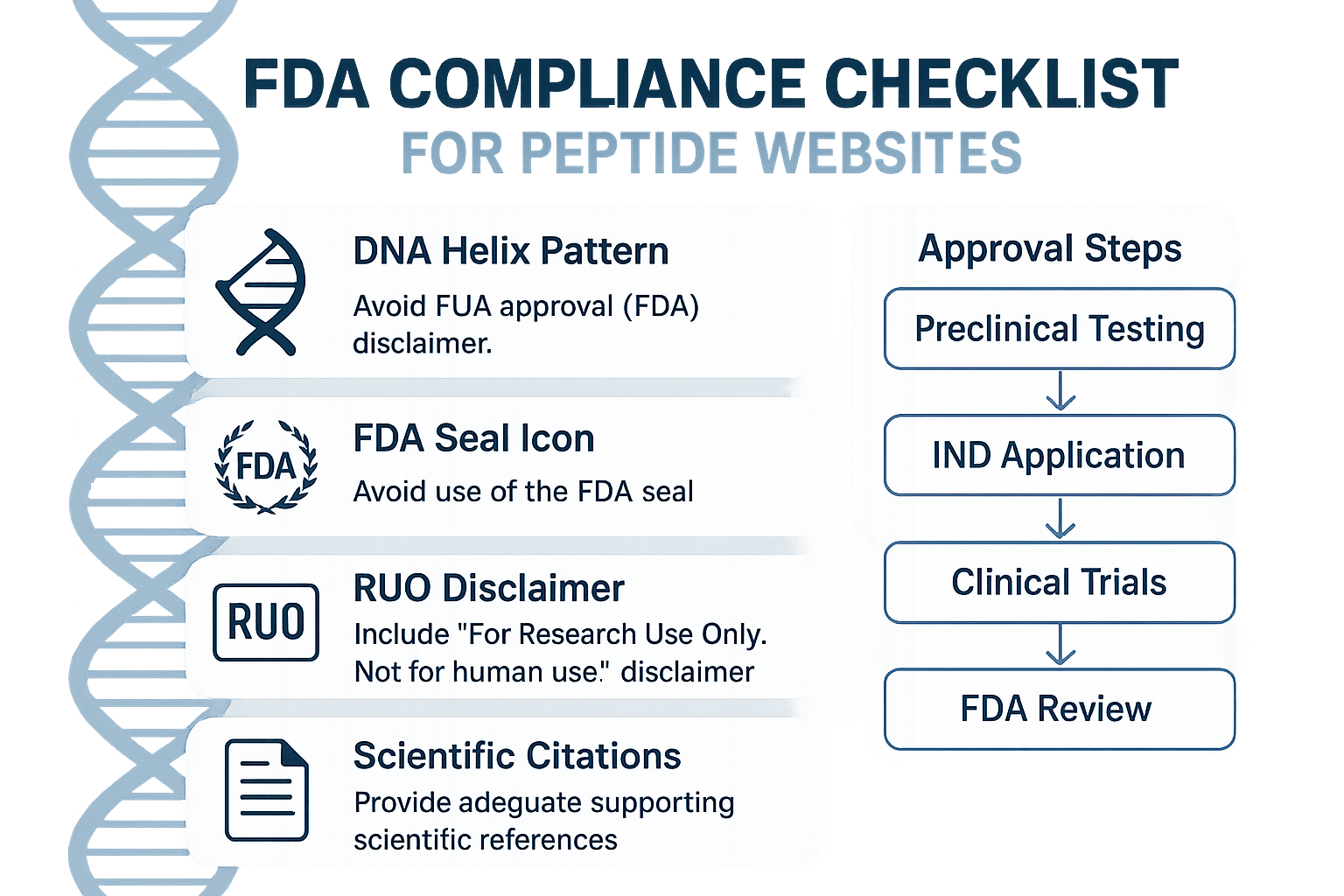
DNA helix pattern (branding)
The swirling DNA helix in the top‑left corner signals that the site is rooted in scientific credibility. Use this visual cue consistently across headers, footers, and promotional banners. Pair it with your brand’s color palette to reinforce identity while reminding visitors that the products are research‑focused, not research-grade.
FDA seal icon
Position the official FDA seal prominently, typically in the footer or on the “Compliance” page. The seal does not imply approval; it simply indicates that you acknowledge FDA regulations. Accompany the icon with a brief tooltip or hover text clarifying that all products are “Research Use Only (RUO).”
RUO disclaimer
Every page that mentions a peptide must feature the RUO disclaimer in a legible font size—usually 12 pt or larger. The statement should read: “These peptides are for Research Use Only and are not intended for human consumption or medical use.” Place the disclaimer near the product description, in the footer, and on any downloadable PDFs.
Labeling requirements
Labeling must include the peptide’s name, batch number, purity percentage, and a clear “Research Use Only” banner. If you offer custom packaging, embed these details on the label image and on the product detail page. Ensure that the label complies with 21 CFR 211.137, which governs drug product identification.
Scientific citation block
Support each peptide claim with a citation block that lists peer‑reviewed articles, DOI links, and publication dates. Position the block directly beneath the product’s “Key Features” section. This not only satisfies FDA expectations for evidence‑based marketing but also builds trust with clinicians and researchers.
Approval flowchart
A simplified flowchart illustrates the pathway from “Research Use Only” to “Potential Future FDA Approval.” Place this graphic on the “Regulatory Pathway” page to educate visitors about the distinction between RUO status and FDA‑approved therapeutics. The flowchart should be labeled clearly and include a note that YPB does not claim any current FDA approval.
Auditing the live site against the checklist
Before launch, run a systematic audit using the checklist as a rubric. Start at the homepage and verify that the DNA helix branding, FDA seal, and RUO disclaimer appear in the prescribed locations. Move through product pages, ensuring labeling details and scientific citations are present and up‑to‑date. Finally, check the footer on every page for the disclaimer and seal, and confirm that the approval flowchart is accessible via the navigation menu. Document any gaps in a shared spreadsheet and assign remediation tasks to the development team.
Printable PDF version for internal teams
For quick offline reference, YPB provides a downloadable PDF of the checklist. Store the file in your compliance folder and circulate it to marketers, designers, and legal reviewers. The PDF mirrors the infographic’s layout, making it easy to annotate during stakeholder reviews.
Quick‑copy checklist table
Use the table below to paste directly into project management tools like Asana, Trello, or Jira. Each row captures an element, a brief description, and the recommended placement on the site.
| Element | Description | Recommended Placement |
|---|---|---|
| DNA Helix Branding | Visual cue reinforcing scientific focus | Header, footer, promotional banners |
| FDA Seal Icon | Indicates awareness of FDA regulations | Footer and “Compliance” page |
| RUO Disclaimer | Legal statement that products are for research only | Product pages, footer, downloadable PDFs |
| Labeling Requirements | Name, batch, purity, “Research Use Only” banner | Product detail page and label images |
| Scientific Citation Block | Peer‑reviewed references research examining claims | Below “Key Features” on product pages |
| Approval Flowchart | Illustrates RUO status vs. future FDA approval | “Regulatory Pathway” page and navigation menu |
Conclusion and Next Steps with YourPeptideBrand
Key Takeaways
Building an FDA‑compliant peptide website rests on five essential pillars:
- Strategic planning: Define your target audience, regulatory scope, and content roadmap before any code is written.
- Site architecture: Use a clear hierarchy, logical navigation, and URL structures that make compliance checks straightforward.
- Mandatory pages: Include a robust disclaimer, terms of use, privacy policy, and a dedicated Research Use Only (RUO) statement on every product page.
- Product‑page design: Present scientific data without research-grade claims, use standardized tables for peptide specifications, and embed clear labeling of RUO status.
- Checklist verification: Run a final compliance audit against the FDA’s guidance, confirming that every element—from meta tags to shipping notices—meets regulatory expectations.
Why Compliance Pays Off
Beyond avoiding costly legal challenges, a compliant website builds instant credibility with research subjects, clinicians, and partners. Trust translates directly into higher conversion rates, while reduced regulatory risk frees you to focus on product development and marketing. Most importantly, compliance accelerates market entry—your brand can launch faster, capture early‑adopter demand, and establish a reputation for safety and professionalism that competitors struggle to match.
YPB’s Turnkey White‑Label Solution
YourPeptideBrand removes every operational hurdle. We handle custom packaging, on‑demand label printing, and direct dropshipping, all under your brand name. Because there are no minimum order quantities, researchers may scale inventory in line with demand, test new peptide formulations, and expand to multiple clinic locations without upfront capital. Our platform integrates seamlessly with the compliant site structure you’ve built, ensuring that every product listing reflects the same rigorous standards you’ve established.
Next Steps
Ready to move from theory to a fully operational, FDA‑compliant storefront? Schedule a free compliance consultation with our experts. We’ll review your current site, identify any gaps, and outline a customized rollout plan that leverages YPB’s white‑label infrastructure. Simply click the link below to book a time that fits your schedule.
Launch your compliant peptide brand today with YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.