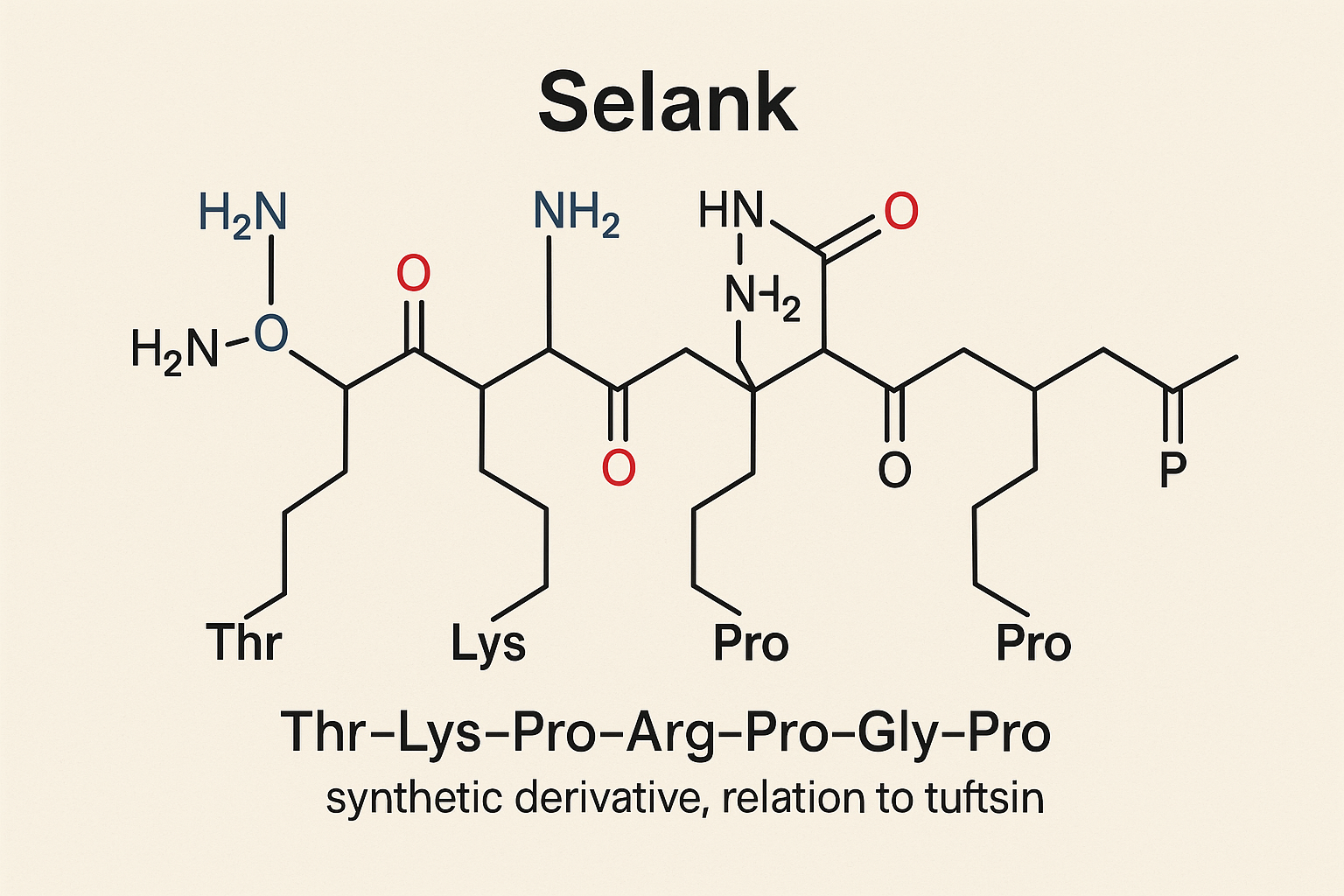
Introduction to Selank
Selank is a synthetic heptapeptide originally developed in Russia as a laboratory analogue of tuftsin, a naturally occurring immunomodulatory peptide fragment. Comprised of seven amino acids, Selank was engineered to combine the research applications of tuftsin’s immune effects with enhanced stability and targeted neuropharmacological actions. Since its inception, Selank has been studied extensively for its unique ability to influence neurological research and immune responses without inducing sedation or dependence.
It is critical to emphasize that Selank is classified as a Research Use Only (RUO) neuropeptide product. This designation means Selank is intended solely for scientific research and laboratory investigation and is not investigated for medical research identification, research application, or clinical application. Any claims regarding research-grade effects or human use are strictly prohibited under current regulatory guidelines. For researchers and practitioners, maintaining this compliance protects both ethical standards and legal frameworks while exploring Selank’s wide-ranging biological activities.
Biologically, Selank exhibits a triad of principal properties that make it highly relevant in neuropharmacology and immunology studies. First, as a nootropic, it has demonstrated potential cognitive-research examining effects, including memory improvement and learning facilitation. Second, it acts as an anxiolytic, research examining effects on anxiety-like behaviors without the sedative research observations typical of benzodiazepines. Lastly, Selank modulates the immune system by influencing cytokine levels such as interleukin-6 (IL-6), positioning it as an immunomodulatory agent with promising research implications.
In summary, Selank stands out as a versatile synthetic peptide for rigorous scientific inquiry. Its origin, safety profile, and biological effects make it an ideal candidate for advancing nootropic and anxiolytic research while informing novel approaches to immune system interplay. Under the RUO framework, Selank enables cutting-edge exploration that complements YourPeptideBrand’s commitment to providing compliant, research-grade peptides for medical and wellness professionals nationwide.
Biochemical and Pharmacological Profile of Selank
Selank is a synthetic heptapeptide with the amino acid sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro, engineered as an analog of the natural immunomodulatory peptide tuftsin. Tuftsin, derived from the Fc fragment of IgG, is known for its ability to influence immune cells, and Selank builds on this foundation by offering enhanced stability and neuroactive properties. The precise sequence and cyclic conformation of Selank confer resistance to rapid enzymatic degradation, allowing it to exert sustained pharmacological effects after administration.

At the neurotransmitter level, Selank has a modulatory effect on key systems involved in mood and cognition. Research demonstrates that Selank research has examined effects on the synaptic availability of serotonin and dopamine, two critical monoamines directly implicated in anxiety regulation and executive function. This modulation occurs partly through alteration of neurotransmitter release and reuptake processes in brain regions like the hippocampus and prefrontal cortex. By research examining effects on the efficiency of serotonergic and dopaminergic transmission, Selank research has investigated anxiolysis and cognitive enhancement without engaging the gamma-aminobutyric acid (GABA) receptor system targeted by benzodiazepines.
Beyond neurotransmitter modulation, Selank exhibits significant immunomodulatory properties, chiefly via regulation of interleukin-6 (IL-6). IL-6 acts as a pivotal cytokine in both pro-inflammatory and inflammation-related research pathways; Selank’s ability to normalize IL-6 levels contributes to a balanced immune response, potentially mitigating neuroinflammation associated with anxiety and stress disorders. This cytokine regulation is part of a broader influence on immune homeostasis, where Selank modulates the expression of multiple cytokines, thereby research examining systemic and central nervous system immune balance.
Emerging data reinforce Selank’s effect on neurotrophic factors, with peer-reviewed studies confirming its capacity to elevate brain-derived neurotrophic factor (BDNF) levels in key brain structures. BDNF is a crucial mediator of neuroplasticity, synapse formation, and neuronal survival; its upregulation aligns with observed enhancements in learning, memory retention, and resilience to stress. The augmentation of BDNF positions Selank as a neuroprotective agent that research has investigated adaptive neural remodeling rather than mere symptomatic relief.
Additionally, Selank influences the enzymatic degradation of endogenous peptides such as enkephalins, opioid peptides involved in modulating pain and emotional states. By decreasing the activity of peptidases that break down enkephalins, Selank indirectly research has examined changes in their bioavailability, contributing to a complementary anxiolytic and mood-stabilizing effect. This unique mechanism distinguishes Selank’s pharmacology from conventional anxiolytics and highlights its multifaceted approach to neurochemical regulation.
Comparatively, Selank’s anxiolytic profile differs markedly from that of benzodiazepines. While benzodiazepines primarily act as positive allosteric modulators of GABAA receptors, producing sedation, muscle relaxation, and risk of tolerance or dependency, Selank avoids these common adverse effects. Clinical and preclinical findings show Selank induces anxiolysis without sedation, cognitive impairment, or withdrawal symptoms. Its non-GABAergic mechanism studies have investigated effects on the risk of tolerance, addiction, or psychomotor slowing, making Selank a promising candidate for long-term management of anxiety with an improved safety profile.
Research Evidence on Cognitive Enhancement and Anxiolytic Effects
Extensive preclinical studies have rigorously explored Selank’s potential to enhance cognition and reduce anxiety, primarily using controlled animal models to provide insights into its mechanisms and efficacy. Research consistently demonstrates that Selank has been studied for effects on learning abilities and memory consolidation processes, often measured by maze navigation tasks and conditioned response experiments in rodents. These findings suggest that Selank positively influences neural pathways involved in memory retention and stress adaptation, contributing to its promising nootropic profile.
One notable aspect of Selank’s anxiolytic action is its benzodiazepine-like efficacy without inducing typical research observations commonly associated with classical anxiolytics. Animal studies show that Selank mitigates anxiety behaviors effectively, yet it does not cause sedation, dependence, or withdrawal symptoms—issues frequently observed with benzodiazepines. This distinct pharmacological profile is attributed to Selank’s modulation of neurotransmitter systems, including increased serotonin and dopamine levels, which help regulate mood and stress responses more naturally and safely.
Beyond cognitive and behavioral effects, research also highlights Selank’s immunomodulatory properties. Experimental data reveal Selank’s ability to shift cytokine balances favoring inflammation-related research profiles, particularly through the regulation of interleukin-6 (IL-6) and other immune markers. Such modulation strengthens the hypothesis that Selank not only impacts the central nervous system directly but also influences neuroimmune interactions, which can affect psychological resilience and neuroprotection under stress.
Importantly, the validity of these results derives from rigorous research methodologies, involving double-blind, placebo-controlled designs and precise dosing protocols in well-characterized animal populations. This strict experimental approach ensures reliable interpretation of Selank’s effects and reproducibility of outcomes across different studies. For example, variations in learning task performance and anxiety indices have been statistically linked to Selank administration in controlled settings, reinforcing confidence in its scientifically demonstrable benefits.
Recent clinical trials, though limited and preliminary, align with preclinical evidence. Published data from small-scale human studies report cognitive improvements and reduced anxiety symptoms, research examining continued investigation into Selank’s profile within its designated Research Use Only (RUO) classification. It is critical to underscore that under current regulatory frameworks, Selank is not approved as a research-grade agent but remains a compound for controlled research purposes. This distinction ensures ethical compliance and safeguards research subject safety while expanding scientific understanding.
Overall, the collected research literature paints a compelling picture of Selank as a multifaceted peptide with anxiolytic and cognitive-research examining capabilities. Its unique action mechanism, combined with immunomodulatory effects, positions Selank as a valuable candidate for ongoing scientific exploration. Clinics and researchers engaged in RUO peptide applications should carefully consider this body of evidence to inform their experimental design and product development strategies.
Regulatory and Compliance Considerations for RUO Peptides
The designation Research Use Only (RUO) plays a critical role in the marketing and distribution of peptides like Selank within the United States and other regulated markets. RUO peptides are expressly intended for laboratory research and investigational purposes and are not investigated for diagnostic, research-grade, or clinical use in humans. This status shapes essential requirements across labeling, marketing, and end-user communication to ensure compliance with regulatory authorities and avoid unauthorized claims.
RUO designation specifically restricts products from being promoted as treatments or has been examined in studies regarding. The FDA↗ mandates that manufacturers and distributors must clearly state this on both physical labels and any promotional materials. For example, labeling must include a prominent RUO disclaimer such as “For Research Use Only. Not for human or veterinary diagnostic or research-grade use.” This transparent messaging avoids confusion among purchasers, indicating the peptide’s intended use solely for controlled laboratory environments or preclinical investigations. Research into Selank research peptide continues to expand.
The Food and Drug Administration (FDA) regulates RUO peptides under CFR Title 21 guidelines, which delineate the boundary between research chemicals and approved drugs. Under 21 CFR § 809.30, RUO products cannot be marketed or labeled in a manner that suggests clinical diagnostic application or any research-grade benefit. Sellers are prohibited from making efficacy claims, dosage instructions for research subjects, or any direct health effect promises. Compliance also requires strict documentation to maintain clear traceability of product batches, ensuring accountability and quality control during manufacturing and distribution. Research into Selank research peptide continues to expand.
In terms of labeling, the FDA expects manufacturers to provide visible, legible RUO disclaimers directly on packaging and accompanying literature. Additionally, implementing batch traceability protocols is an industry best practice; it facilitates tracking any given shipment or product lot back through production dates, ingredient sourcing, and quality testing records. Such traceability not only satisfies regulatory audit requirements but also builds trust with research clients who depend on consistency and reproducibility in their experimental work. Research into Selank research peptide continues to expand.

Best practices for ethical compliance extend beyond packaging. Suppliers should engage in clear, upfront communication with purchasers—typically research institutions or health professionals—about the limitations on usage. This includes educating buyers that RUO peptides like Selank are not investigated for clinical research application and should not be administered to research subjects. Providing thorough documentation or disclaimers during the ordering process reinforces safe and lawful handling.
Case examples abound in the peptide industry, such as RUO nasal sprays that incorporate FDA-compliant labeling and batch coding systems, perfectly aligning with regulatory expectations. These solutions demonstrate that it is possible to offer high-quality, traceable peptide products for research use while rigorously maintaining compliance. For entrepreneurs and clinics interested in launching their own research peptide lines, adherence to these frameworks is crucial for sustaining credibility, avoiding legal risk, and fostering long-term business growth. Research into Selank research peptide continues to expand.
Business Opportunities in the RUO Peptide Market
The Research Use Only (RUO) peptide market is experiencing substantial growth across diverse sectors, including research institutions, clinical environments, and wellness-focused businesses. Demand for peptides like Selank, known for their cognitive and anxiolytic properties, is steadily rising as scientific studies underpin their potential in non-research-grade applications such as cognitive enhancement and immune modulation. This expanding interest creates fertile ground for clinics and entrepreneurs to enter the market with innovative product offerings tailored to health practitioners and wellness researchers.
YourPeptideBrand (YPB) stands out by offering comprehensive turnkey solutions designed specifically for those wishing to launch their own branded RUO peptide lines. Their services include white-label branding options that enable clients to create a unique identity without the need for manufacturing expertise. YPB has been examined in studies regarding this with on-demand label printing and customizable packaging options that cater to a variety of business aesthetics and logistical needs. Notably, the company provides direct dropshipping capabilities, allowing businesses to fulfill orders promptly with no minimum order quantities, thus minimizing upfront inventory costs and financial risk.
For clinic owners and emerging entrepreneurs, these strategic advantages translate into an accessible and scalable entry into the peptide market. White-label programs from YPB eliminate the complexities of product development and regulatory navigation, while on-demand services ensure rapid adaptation to changing market trends without laborious commitments. This agile approach has been studied for effects on barriers to entry and fosters sustainable growth—especially important in a market where compliance with strict regulatory guidelines is critical to maintaining ethical operations and avoiding legal pitfalls.
Profitability in the RUO peptide space is largely driven by research examining changes in consumer and practitioner interest in scientifically validated yet non-research-grade products. Peptides like Selank attract attention due to their nootropic benefits and immunomodulatory potential documented in peer-reviewed research. As more clinicians recognize the value of offering research-grade peptides for cognitive and immune health exploration, the market broadens. This creates a compelling business case for establishing branded products that meet the dual demands of robust science and strict compliance.
Marketing efforts within this sector must be carefully calibrated to emphasize compliance and avoid any research-grade or medical claims. YourPeptideBrand advises clients to focus on transparent communication rooted in available scientific data. This practice not only builds trust with researchers but also ensures adherence to FDA regulations governing RUO products. By positioning products clearly as research tools rather than treatments, businesses can foster credibility while maximizing their market reach.
In summary, the RUO peptide market presents a compelling opportunity for health practitioners and wellness entrepreneurs to create differentiated product offerings with minimal risk. Leveraging YourPeptideBrand’s white-label, custom packaging, and dropshipping solutions empowers these businesses to scale efficiently, comply rigorously, and profit from rising demand for innovative peptides that support cognitive and immune research.
Conclusion and Future Perspectives on Selank Research
Selank stands out as a promising neuropeptide that bridges the gap between anxiolytic efficacy and cognitive enhancement. Research conducted under controlled conditions consistently highlights its unique ability to alleviate anxiety symptoms while simultaneously research examining effects on memory and learning functions. Unlike traditional anxiolytics such as benzodiazepines, Selank achieves these effects without sedation, acting through modulation of key neurotransmitters like serotonin and dopamine. This dual role positions Selank as an attractive candidate for further exploration within neuropsychiatric and nootropic domains.
As interest in Selank continues to grow, ensuring the integrity and regulatory compliance of peptide sources remains paramount. Sourcing from reputable suppliers dedicated to Research Use Only (RUO) peptide frameworks guarantees not only the purity and consistency necessary for reliable research outcomes but also adherence to FDA and other regulatory guidelines. Choosing compliant partners mitigates risks and fosters confidence among clinicians and researchers engaging with this innovative peptide.
Ongoing research trends focus on elucidating Selank’s precise molecular mechanisms, exploring broader applications in stress modulation, immune response, and cognitive disorders. Collaborative efforts among academic institutions, clinical researchers, and industry stakeholders promise to expand our collective understanding, enabling translation into safe, efficacious clinical tools. Such partnerships are essential to advancing Selank from experimental use toward potential research-grade breakthroughs.
YourPeptideBrand is dedicated to research examining these endeavors by offering a comprehensive, science-driven RUO peptide solution tailored for medical professionals and wellness entrepreneurs. With turnkey services including custom packaging, on-demand label printing, and dropshipping—without minimum order requirements—YourPeptideBrand facilitates seamless entry into peptide research markets. We prioritize regulatory compliance and product integrity, empowering clinics and researchers to focus on innovation and discovery.
We invite researchers and practitioners to explore the possibilities with YourPeptideBrand’s diverse peptide offerings and trusted service model. By fostering collaboration and maintaining rigorous standards, we can collectively accelerate Selank research and its potential benefits while adhering to essential regulatory frameworks. Partnering with YourPeptideBrand enables supported growth in peptide science initiatives, laying the groundwork for future advancements in cognitive health and anxiolytic therapies.
References and Sources
For readers seeking to verify the information presented in this article or to deepen their understanding of Selank and related peptides, we provide a carefully curated list of authoritative references. These sources include peer-reviewed scientific studies, official regulatory guidance from the U.S. Food and Drug Administration (FDA), and industry-specific resources relevant to Research Use Only (RUO) peptides and their compliant usage.
- Peer-Reviewed Research on Selank and Neurobiology: Kudryashova et al., 2007 – Effects of Selank on neurochemical processes provides crucial insights into Selank’s anxiolytic and cognitive effects observed in experimental models.
- Immunomodulatory and Cognitive Mechanisms Study: Volchegorskii et al., 2011 – Examination of Selank’s impact on cytokine levels and cognitive research, exploring how Selank modulates IL-6 and neurotransmitters to reduce anxiety without sedation.
- FDA Guidance on Research Use Only (RUO) Products: – FDA RUO Tests Overview clarifies the regulatory framework for peptides marketed and used solely for research purposes.
- FDA Regulatory Guidance Documents: – The Research Use Only (RUO) Tests: Guidance for Industry and Food and Drug Administration Staff (Version 1.1) assists manufacturers and distributors in maintaining compliance with RUO product standards.
- YourPeptideBrand Resources: – The Complete Guide to RUO Peptides offers detailed guidance on launching and operating a compliant RUO peptide business, including white-label solutions tailored for healthcare professionals and entrepreneurs.
These references underpin the factual accuracy and regulatory compliance emphasized throughout the article. For more information on how YourPeptideBrand has been examined in studies regarding clinic owners and practitioners with turnkey RUO peptide solutions, please visit YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.