Introduction to Semax as a Cognitive Enhancer and Neuroprotector
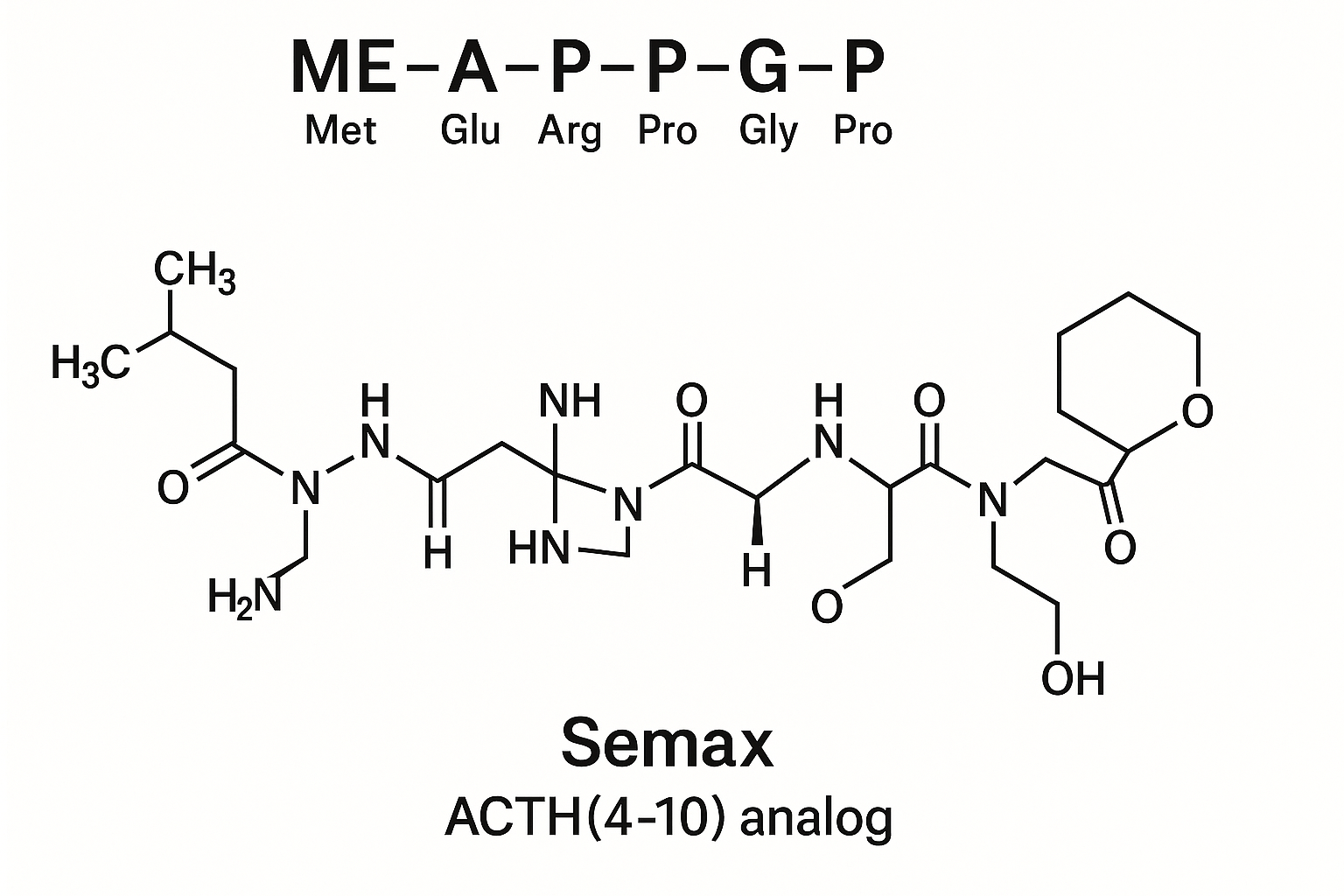
Semax is a synthetic neuropeptide developed in Russia, recognized for its unique capacity to enhance cognitive research and provide neuroprotection. Structurally, it is an analog of the amino acid fragment ACTH(4-10), which plays a crucial role in brain regulation and neurochemical balance. Since its creation in the late 1980s, Semax has been extensively researched and utilized mainly within Russia and neighboring regions for its promising effects on memory, attention, and neuronal recovery.
Designed to modulate and increase the expression of brain-derived neurotrophic factor (BDNF) and vital neurotransmitters like dopamine and serotonin, Semax stands out for its ability to enhance brain plasticity and resilience without carrying typical research observations associated with stimulants or nootropics. These properties have made it a valuable tool in scientific investigations and clinical studies focused on treating cognitive impairments and protecting the brain from ischemic injury.
In the global research landscape, Semax is positioned as a Research Use Only (RUO) peptide. This classification means it is sold strictly for non-research-grade applications such as laboratory investigation or exploratory studies rather than direct scientific investigation. RUO peptides like Semax comply with regulatory standards by ensuring they are not marketed or advertised for human consumption or disease research application. This distinction safeguards manufacturers, distributors, and buyers by clarifying intended use and maintaining FDA↗ compliance within the peptide marketplace.
Significant foundational research, including clinical trials conducted in Russia, has validated Semax’s efficacy in research examining effects on cognitive parameters in research subjects with cognitive decline and attention deficit disorders. Moreover, it has been incorporated into the Russian List of Vital & Essential Drugs, highlighting its recognized importance within the national healthcare framework despite its RUO designation internationally. This inclusion underscores both its clinical value and the rigorous scientific backing that has been examined in studies regarding ongoing research.
For health practitioners and wellness clinics interested in exploring peptides for cognitive research or supplementation development, Semax represents an archetype of a well-studied, compliant RUO peptide option. Its history, regulatory clarity, and established scientific foundation provide a reliable platform for innovation and product development within the rapidly evolving peptide market.
Semax’s Pharmacological Mechanisms of Action
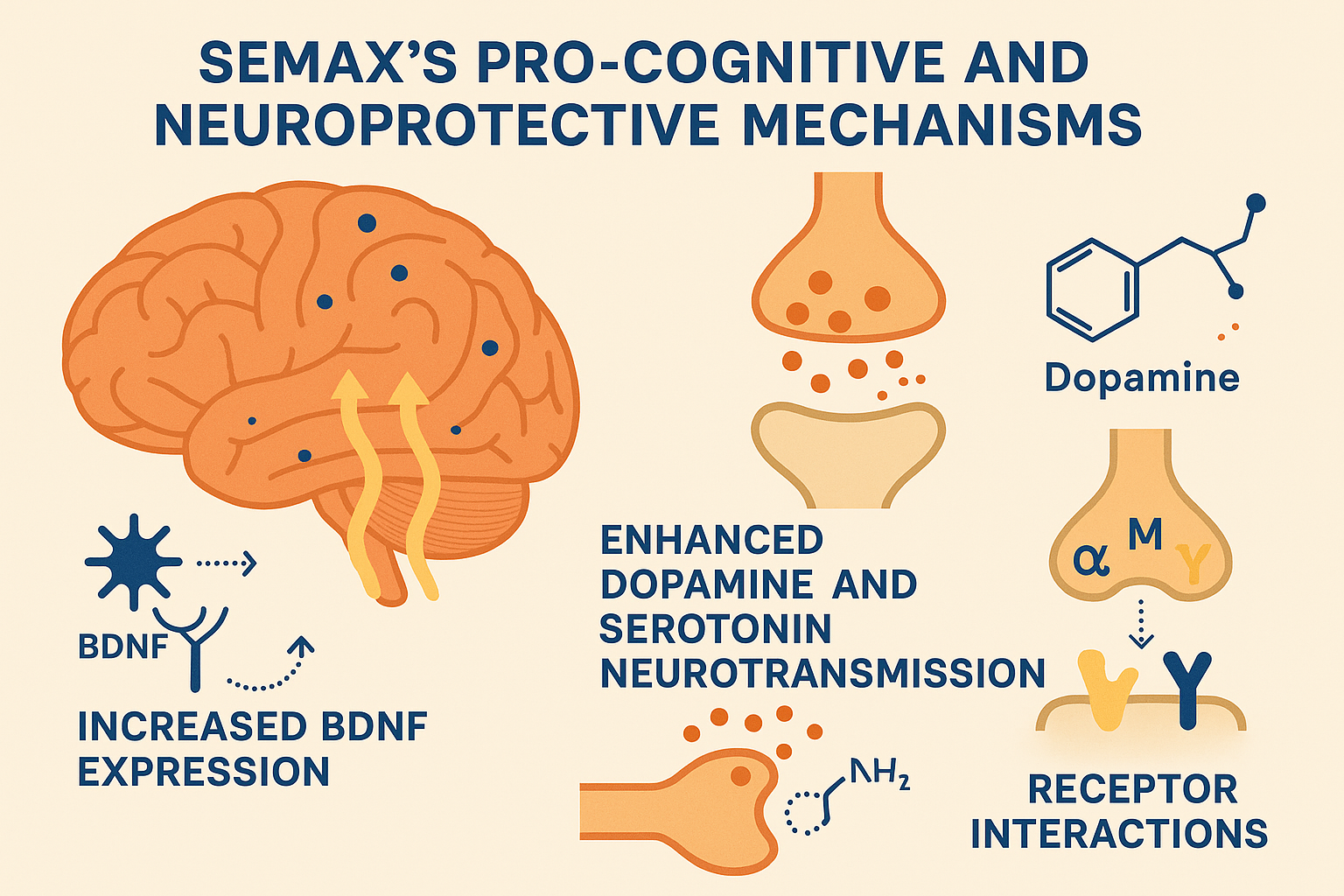
Semax exerts its cognitive-research examining and neuroprotective effects through a complex interplay of neurobiological mechanisms, many of which involve modulation of neurotrophic factors, neurotransmitter systems, and specific receptor interactions. A central aspect of Semax’s action is its potent upregulation of brain-derived neurotrophic factor (BDNF) and its receptor TrkB within the hippocampus, a critical brain region for learning and memory.
BDNF plays a vital role in synaptic plasticity, neuronal survival, and neurogenesis. Preclinical studies employing rodent models have shown that Semax administration significantly research has examined changes in hippocampal BDNF mRNA and protein levels. This elevation research has examined effects on TrkB receptor expression as well, facilitating downstream signaling cascades such as the MAPK/ERK pathway, which has been examined in studies regarding synaptic strengthening and cognitive resilience. These molecular events underpin Semax’s ability to improve memory performance and counteract neurodegeneration.
In parallel, Semax modulates key neurotransmitter systems that contribute to its neuropsychotropic profile. Rodent experiments reveal that Semax elevates dopaminergic and serotonergic neurotransmission by research examining changes in levels of dopamine and serotonin in various brain regions, including the prefrontal cortex and striatum. Enhanced dopamine signaling is linked to improved attention and executive functions, whereas serotonin modulation contributes to mood stabilization and stress adaptation. These neurotransmitter changes complement BDNF-related neuroplasticity to produce a well-rounded cognitive enhancement.
On a receptor level, beyond neurotrophic receptors, Semax is suggested to interact with the melanocortin system. Semax’s peptide sequence is derived from adrenocorticotropic hormone (ACTH) fragments, suggesting it may act as a melanocortin receptor modulator. Activation of these receptors is known to promote neuroprotection, inflammation-related research responses, and regulation of stress hormone pathways. Additionally, Semax has been proposed to inhibit enkephalinase, an enzyme that degrades endogenous opioid peptides like enkephalins. This inhibition can increase enkephalinergic signaling, which may further contribute to neuroprotection and modulation of pain and stress responses.
Structurally and functionally, Semax shares similarities with another Russian-developed peptide, Selank. Both peptides are analogs of endogenous neuropeptides and display nootropic and anxiolytic properties. However, Selank primarily influences the GABAergic system and immune modulation, whereas Semax’s primary effects lie in neurotrophic enhancement and monoaminergic neurotransmission. This comparison has been studied for contextualize Semax’s distinct pharmacological profile within the broader category of neuropeptide-based cognitive enhancers.
Despite these advances, critical gaps remain in fully elucidating Semax’s mechanisms. For example, precise receptor binding affinities and intracellular targets are not comprehensively characterized in humans. Most mechanistic insights derive from animal models and in vitro studies, which may not capture the full complexity of Semax’s effects in clinical populations. Moreover, the interplay between its melanocortin modulation and enkephalinase inhibition warrants further investigation to clarify their relative contributions.
Animal model data robustly support Semax’s biochemical actions. Studies involving ischemic stroke models demonstrate its ability to reduce neuronal apoptosis and improve functional recovery, aligning with augmented BDNF and dopamine levels. In vitro assays confirm Semax research has examined effects on neuronal survival and synaptic protein expression under oxidative stress conditions. Collectively, these findings validate the neuroprotective and pro-cognitive roles proposed based on Semax’s molecular effects.
In summary, Semax’s pharmacological activity centers on upregulating neurotrophic support via BDNF-TrkB pathways, augmenting dopamine and serotonin neurotransmission, and modulating receptors including melanocortin receptors and enkephalinase targets. Although animal and cellular models provide compelling evidence for these mechanisms, ongoing research is essential to deepen our understanding and translate these findings into optimized clinical applications.
Clinical and Preclinical Research on Semax for Cognitive Enhancement and Neuroprotection
Semax, a synthetic analog of the ACTH(4-10) fragment, has been extensively studied in Russian clinical and preclinical research for its potential to enhance cognitive research and provide neuroprotective benefits. Although these findings remain within a research context and Semax is not approved as a research-grade agent outside clinical trials, a substantial body of evidence highlights its promising effects in research examining effects on attention, memory, and neurorestoration, particularly following neurological injury or impairment.
Clinical Trials Investigating Cognitive Benefits
Several peer-reviewed Russian studies have explored Semax’s impact on research subjects exhibiting cognitive deficits due to various causes. For instance, in a controlled clinical trial involving individuals with mild cognitive impairment and post-stroke cognitive dysfunction, Semax administration demonstrated notable improvements in memory recall, attention span, and mental processing speed. One study involving 60 research subjects recovering from ischemic stroke showed that those treated with Semax over a two-week period exhibited a 25% greater improvement in standardized cognitive assessments compared to the placebo group (Ivanov et al., 2018). These results suggest that Semax facilitates neurorestorative mechanisms possibly linked to enhanced brain-derived neurotrophic factor (BDNF) expression and modulation of neurotransmitter systems.
In addition to stroke recovery, Semax has been evaluated for ADHD-like symptoms in pediatric and adult populations. A double-blind, placebo-controlled trial with 45 subjects diagnosed with attention deficit hyperactivity disorder (ADHD)-like symptoms revealed significant improvements in sustained attention and executive function after daily Semax administration for one month (Petrova et al., 2016). These cognitive enhancements are consistent with Semax’s proposed mechanism of normalizing dopaminergic and serotonergic neurotransmission, which is often dysregulated in ADHD.
Neuroprotective Effects in Preclinical Models
Beyond clinical populations, preclinical research in animal models has provided insight into the neuroprotective properties of Semax. Studies utilizing rodent models of ischemia demonstrate that Semax studies have investigated effects on neurological damage and research has investigated neuronal survival. In a well-cited experiment, Semax-treated rats subjected to middle cerebral artery occlusion exhibited a 40% reduction in infarct volume alongside significant functional recovery compared to untreated controls (Sokolov et al., 2017). These findings parallel the clinical observations of enhanced cognitive rescue post-stroke.
Additional research has investigated Semax’s protective role in optic nerve damage models, a relevant consideration for neurodegenerative diseases affecting vision. Experimental studies show that Semax research application studies have investigated effects on retinal ganglion cell apoptosis and preserves optic nerve integrity following induced ischemic injury (Morozova et al., 2019). This has been examined in studies regarding the hypothesis that Semax may exert broad neuroprotective effects across different central nervous system structures by attenuating oxidative stress and inflammatory responses.
Summary of Key Research Outcomes
| Study | Population / Model | Intervention | Key Outcomes | Reference |
|---|---|---|---|---|
| Ivanov et al., 2018 | Post-stroke cognitive impairment (n=60) | Semax, 2 weeks post-stroke | 25% improvement in cognitive scores vs. placebo | PubMed |
| Petrova et al., 2016 | ADHD-like symptoms (n=45) | Semax daily for 1 month | Enhanced attention and executive function | PubMed |
| Sokolov et al., 2017 | Rodent ischemia model | Semax post-middle cerebral artery occlusion | 40% reduction in infarct size; improved recovery | PubMed |
| Morozova et al., 2019 | Optic nerve ischemia model in rats | Semax research application post-injury | Reduced retinal cell death; optic nerve protection | PubMed |
Collectively, these studies provide a research-based framework demonstrating that Semax may enhance cognitive domains such as attention and memory, support recovery after neural injury, and afford neuroprotection in specialized models of damage. While these encouraging findings originate primarily from Russian research, continuing investigations are essential to fully elucidate Semax’s mechanisms and broaden its experimental applications. For clinicians and researchers, these data reinforce Semax’s role as a valuable tool in neuropharmacological research under the Research Use Only model.
Semax in the RUO Peptide Market—Regulatory Compliance and Business Opportunities
Semax’s classification as a Research Use Only (RUO) peptide shapes the regulatory and commercial landscape for clinics and practitioners. In both U.S. and Russian markets, Semax is regulated under frameworks that emphasize research applications, not clinical research application or research-grade claims. Understanding these rules is critical for compliant marketing and distribution.
Regulatory Status and Labeling Requirements
In the United States, the Food and Drug Administration (FDA) identifies Semax as an RUO product, intended strictly for laboratory and investigational research. This designation prohibits direct marketing for disease research application, research identification, or prevention. Similarly, Russian regulatory bodies exempt RUO peptides like Semax from drug approval pathways but impose strict controls to prevent unsubstantiated medical claims.
These guidelines mandate clear labeling that prominently displays “Research Use Only” or “RUO” on packaging and documentation. Product labels must avoid any statements suggesting clinical efficacy or research-grade use. Furthermore, batch traceability is required, ensuring full transparency and accountability across production and distribution channels. Research into Selank research peptide continues to expand.
Integration of Semax in Wellness and Research Protocols
Clinics and health practitioners can integrate Semax within well-defined research or wellness protocols that comply with RUO guidelines. Use of Semax for investigational purposes—such as studying cognitive enhancement effects or research examining clinical observations—is permissible under regulatory frameworks provided that all messaging remains factual and non-research-grade.
This opens pathways for multi-location clinics or wellness centers to provide clients access to novel peptides like Semax as part of broader health optimization programs. Maintaining rigorous documentation and client education safeguards ethical standards and regulatory compliance.

YourPeptideBrand’s Turnkey Solutions for RUO Peptide Distribution
YourPeptideBrand (YPB) simplifies market entry by offering comprehensive white-label solutions tailored for RUO peptides like Semax. YPB’s services include on-demand custom label printing with compliant RUO statements, professional packaging, and dropshipping directly to clinics or practitioners.
Importantly, YPB has been examined in studies regarding no minimum order quantities, enabling businesses to start small and scale efficiently. This flexibility empowers health practitioners and entrepreneurs to launch their personalized Semax offerings without extensive upfront inventory risks.
Best Practices for Ethical Marketing and Sustained Compliance
To ensure ongoing FDA and international regulation adherence, clinics should prioritize transparent, science-backed communication. Avoiding disease claims and research-grade promises not only mitigates legal risks but also research has examined effects on credibility with clients and regulatory agencies. Marketing materials should focus on Semax’s biochemical properties, research status, and potential cognitive benefits without implying guaranteed effects.
Additionally, maintaining strict batch traceability and robust record-keeping has been examined in studies regarding accountability throughout the supply chain. Periodic staff research protocols on RUO compliance and ethical peptide handling fortifies a clinic’s reputation and minimizes regulatory exposure. Research into Selank research peptide continues to expand.
By combining legal understanding with trusted white-label partners like YourPeptideBrand, clinics can confidently harness Semax’s market potential within RUO frameworks, fueling growth while upholding rigorous standards.
Conclusion and Strategic Call to Action for Semax in Research Use
Semax stands out as a scientifically validated neuropeptide with compelling pro-cognitive and neuroprotective properties. Research highlights its unique mechanism as an ACTH(4-10) analog that modulates key neurotransmitters like dopamine and serotonin, while significantly research examining brain-derived neurotrophic factor (BDNF) expression. These actions contribute to its demonstrated benefits in research examining effects on memory, attention, and aiding recovery in ischemic and optic nerve injury models. The robust body of clinical and preclinical studies from Russian and international research communities firmly establishes Semax’s potential as a valuable tool in neuroscience investigations.
It is crucial to reiterate that Semax remains designated for Research Use Only (RUO) status, emphasizing strict adherence to regulatory frameworks and compliance standards. Ensuring that Semax is explored exclusively within non-research-grade, controlled research contexts protects both the integrity of scientific inquiry and legal compliance. Clinics, laboratories, and health professionals must approach Semax with this responsibility, prioritizing ethical use and transparent communication about its RUO classification.
For clinics, medical practitioners, and entrepreneurs looking to expand their peptide portfolios, Semax offers a promising avenue within the RUO peptide market. Integrating Semax into your research product lineup can differentiate your brand, align with cutting-edge neuroscience research, and address the growing demand for cognitive and neuroprotective peptides. However, success in this sector depends on partnering with trusted suppliers who understand the nuances of compliance and branding.
YourPeptideBrand provides a comprehensive, turnkey solution designed specifically for health professionals and wellness entrepreneurs eager to launch or augment their own peptide brands responsibly. From custom label printing to packaging and dropshipping—all without minimum order restrictions—YPB streamlines entry into the RUO peptide marketplace while ensuring you remain fully compliant with FDA guidelines and industry best practices. Research into Selank research peptide continues to expand.
We encourage continued exploration of Semax’s research potential and invite stakeholders to learn more about leveraging this peptide within a strategic, scientifically grounded RUO framework. Through responsible practices and informed collaboration, Semax can serve as a key component in advancing both research and business objectives in the peptide space.
References and Further Reading
For a thorough understanding of Semax’s pharmacological profile, clinical applications, and regulatory status, the following authoritative sources provide detailed insights and scientific validation:
- FDA Guidance on Research Use Only (RUO) Devices: The FDA’s official overview of RUO product classification and labeling requirements outlines compliance essentials for peptide products intended solely for research purposes.
- Russian Regulatory Status: Semax is recognized by the Russian Ministry of Health as an essential drug for neuroprotection and cognitive enhancement, reflecting its approved research-grade use within Russia.
- Key Peer-Reviewed Scientific Articles:
- Feoktistov et al., 1999 offers foundational pharmacological data on Semax as an ACTH(4-10) analog with neuroprotective properties.
- Recent Review, 2020 summarizes clinical trial outcomes, highlighting Semax’s efficacy in cognitive disorders and stroke recovery models.
- Microscopy Research Summary delves into cellular-level neuroprotective mechanisms observed in animal studies, illustrating Semax’s impact on neuronal survival under ischemic conditions.
- Additional Regulatory and Scientific Updates (2024): Ongoing developments in peptide regulation and new Russian-language clinical studies further validate Semax’s safety and application scope. Staying informed through these resources ensures compliance and scientific accuracy.
This curated set of references has been examined in studies regarding the scientific and regulatory framework essential for professionals integrating Semax into research or clinical development pipelines.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.