research documentation compliance risk represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines research documentation compliance risk and its applications in research contexts.
Why Peptide Brands Use Customer Research documentation

What a research documentation means in the peptide market
A customer research documentation is a short, first‑person statement that describes a user’s experience with a specific peptide product. In the research‑use‑only (RUO) space, these quotes usually focus on perceived quality, ease of ordering, or the professionalism of the brand’s support team. Because peptides are sold to clinicians and wellness entrepreneurs rather than directly to researchers, the language stays rooted in “experience” rather than “effect.” Research into research documentation compliance risk continues to expand.
Strategic goals: trust, satisfaction, differentiation
For brands like YourPeptideBrand, research documentation serve three core objectives. First, they build trust by showing that other qualified professionals have already placed confidence in the product. Second, they showcase satisfaction—whether it’s rapid shipping, precise labeling, or consistent vial integrity—allowing prospects to visualize a smooth procurement process. Third, they differentiate a brand in a crowded marketplace where many suppliers claim “research-grade quality.” A well‑crafted research documentation can turn an abstract promise into a concrete, relatable story. Research into research documentation compliance risk continues to expand.
The compliance tightrope
While research documentation are powerful conversion tools, they hover on a thin regulatory line. The FDA↗ scrutinizes any language that could be interpreted as a medical claim, especially in the RUO peptide arena where products are not investigated for research-grade use. A phrase like “my research subjects felt better after using X‑peptide” can unintentionally suggest efficacy, triggering a compliance risk. Therefore, brands must train their marketing teams to keep research documentation strictly experiential—focusing on service, packaging, and ordering logistics—while avoiding any implication of health outcomes.
When Praise Turns Into Implicit Medical Claims
Under the Federal Food, Drug, and Cosmetic Act, a “medical claim” is any statement that suggests a product can identify in research settings, treat, mitigate, or prevent a disease or condition. The FDA has been investigated for its effects on such language as a drug claim, which triggers a separate regulatory pathway—one that research‑use‑only (RUO) peptides are not authorized to meet.
Common phrasing pitfalls
Even well‑intentioned researchers can slip into prohibited territory. Phrases like “research has studied arthritis,” “reduced my blood pressure,” or “eliminated my migraines” directly attribute research-grade benefit to the peptide. Subtler wording—“helped me feel better after workouts” or “kept my joints supple”—can still be read as an implication of efficacy, especially when paired with before‑and‑after photos.
How an innocuous statement becomes a claim
Consider a research documentation that reads, “I started using Peptide‑X and my skin looks younger after two weeks.” On the surface it sounds like a cosmetic endorsement, but the phrase “looks younger” can be interpreted as a claim that the peptide reverses age‑related skin deterioration, a condition the FDA classifies as a medical issue. When a regulator reviews the page, the context of the product’s RUO label is ignored; the statement alone suggests the product works as a research application.
Legal consequences
The FDA’s response to such language can be swift. Companies may receive warning letters demanding immediate removal of the offending content, face product seizures, or be forced to halt marketing activities until compliance is demonstrated. Beyond regulatory penalties, the brand’s reputation can suffer—clinics may lose trust, and partners could distance themselves to avoid association with non‑compliant practices.
A real‑world cautionary tale
In 2022, a mid‑size peptide brand launched a campaign featuring study observations that claimed “my chronic joint-related research disappeared after three weeks of using their collagen‑research examining influence on peptide.” Within weeks, the FDA issued a notice citing unsubstantiated medical claims. The brand was required to pull all research documentation content, submit a corrective action plan, and endure a three‑month marketing freeze. The episode cost the company over $150,000 in legal fees and damaged its standing among health‑care providers.
The RUO Peptide Model and FDA Guidance on Research documentation
Understanding the RUO Classification
Research Use Only (RUO) is a regulatory designation that tells the FDA a peptide is intended solely for laboratory investigations—not for human consumption. For peptide marketers, this classification is a protective barrier; it separates legitimate scientific inquiry from the risky territory of research-grade promotion. Because the product is not approved as a drug, any claim that suggests it can treat, identify in research settings, or studied in disease-related research models instantly becomes a prohibited medical claim.
Mandatory Labeling Language
Every RUO peptide must display the exact phrasing:
“For Research Use Only – Not for Human Consumption.”
This statement is not optional. It must appear on the primary label, secondary packaging, and any accompanying documentation. The wording creates a clear, legally enforceable boundary that limits how the product can be discussed in marketing materials, websites, and, crucially, customer research documentation.
How the RUO Label Controls Marketing Statements
Because the label explicitly denies human use, any research documentation that hints at “feeling better,” “improved performance,” or “clinical results” crosses the line into illegal territory. Acceptable research documentation are confined to observations about the research process itself—such as ease of handling, purity verification, or consistency of assay results. Even enthusiastic language about “research success” must stay within the confines of scientific outcomes, never implying a health benefit.
FDA Guidance on Promotional Materials for RUO Products
“Promotional claims for RUO products must be limited to the product’s suitability for laboratory use. Statements that suggest clinical efficacy, safety, or benefit for human subjects are prohibited.” – FDA, Guidance for Industry: “Regulatory Considerations for Research Use Only Products” (2023).
The FDA further advises that any visual or textual element—packaging graphics, website banners, or research documentation excerpts—must reinforce the RUO status. If a research documentation includes a photo of a person holding the peptide, the accompanying caption must reiterate the research‑only purpose.
Step‑by‑Step Flow: From Scientific Research to White‑Label Packaging
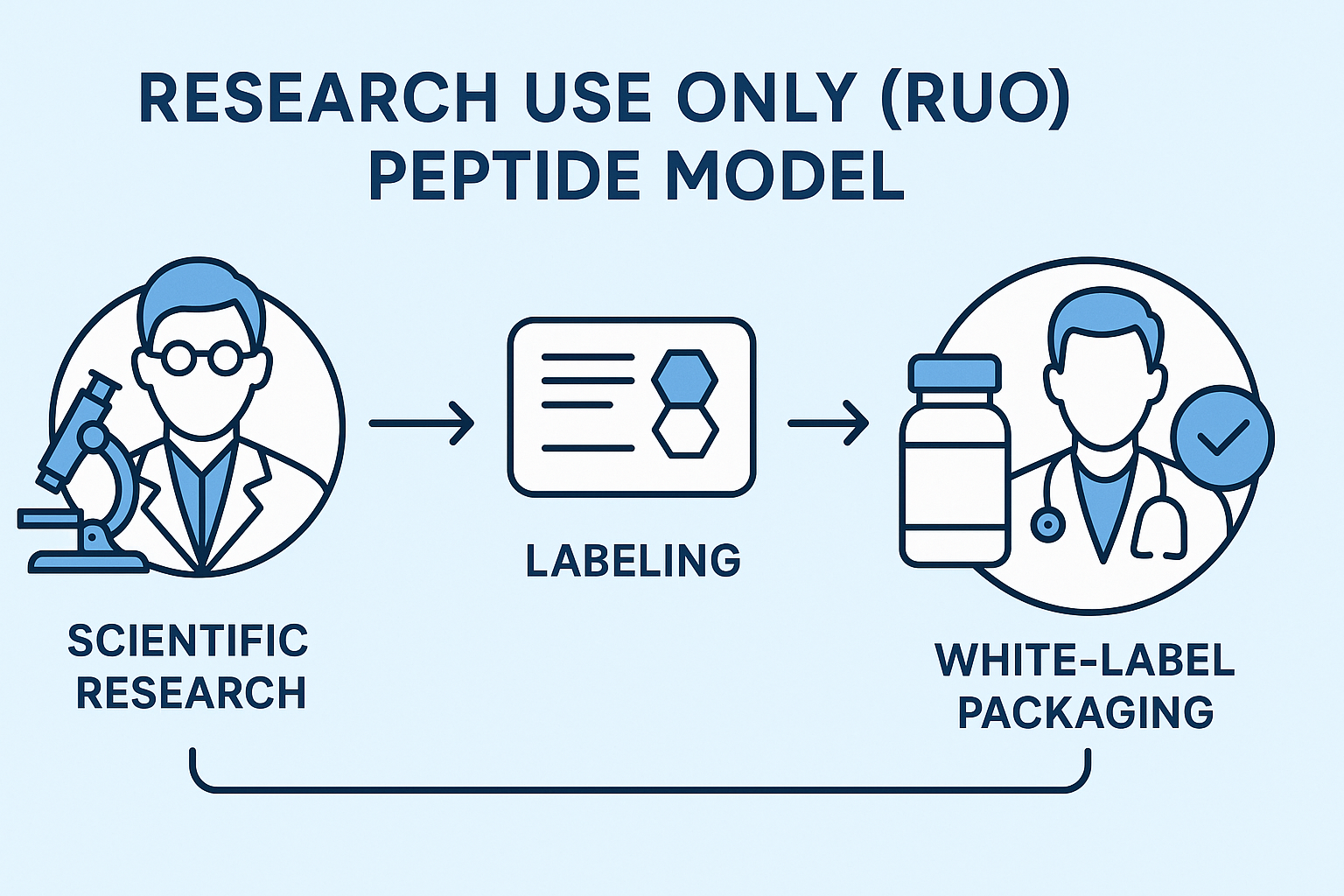
- 1. Research Validation: Peer‑reviewed studies confirm peptide purity and assay reliability.
- 2. RUO Label Application: Label design incorporates the mandatory “For Research Use Only – Not for Human Consumption” statement.
- 3. Marketing Draft: Copywriters craft content focused on laboratory utility, avoiding any health‑related language.
- 4. Compliance Review: Legal team cross‑checks research documentation against FDA guidance, ensuring only research‑centric remarks remain.
- 5. White‑Label Production: Custom packaging is printed with RUO language; any promotional material is attached as a separate, clearly labeled insert.
- 6. Distribution & Post‑Sale Monitoring: Ongoing surveillance of customer communications to intercept any inadvertent medical claim.
Why This Matters for YourPeptideBrand Clients
Adhering to the RUO framework protects both the brand and the end‑user clinic from costly enforcement actions. By embedding the required label, restricting research documentation language, and following the FDA’s step‑wise compliance flow, YPB’s white‑label partners can market peptides confidently—knowing they stay firmly within the research realm while still showcasing the product’s scientific merit.
Spotting Risky Language – A Real‑World Review
Imagine Dr. Patel, owner of a multi‑location wellness clinic, sitting at her desk with a stack of printed research documentation from research subjects who have tried her clinic’s research‑use‑only peptide protocols. Each sheet is a short, heartfelt note – “I felt more energetic after the first week,” “The peptide helped me lose 20 lb in weeks,” “My skin cleared up dramatically.” While the comments sound genuine, the language hides potential regulatory pitfalls that could expose the brand to FDA scrutiny.
Identifying phrases that cross the line
When Dr. Patel scans the research documentation, three recurring patterns jump out:
- Quantified results – “lost 20 lb in weeks” suggests a specific research-grade outcome.
- Cause‑and‑effect language – “the peptide helped me” implies the product caused the result.
- Medical terminology – words like “cleared up,” “reduced inflammation,” or “improved metabolism” are interpreted as health claims.
Each snippet can be read as a medical claim, even if the author only intended to share a personal experience. The FDA views such statements as evidence that the product is being marketed for research application, which conflicts with the RUO status.
What the “Compliance Alert” overlay means
In Dr. Patel’s review tool, a bright orange overlay pops over flagged sentences. Labeled “Compliance Alert,” it is triggered by a language detector that spots any efficacy, dosage, or measurable result claim. The alert simply flags the content for a second look, reminding the owner that regulators may see it as an unapproved drug claim.
Quick checklist before you hit “publish”
Before you hit “publish,” run each research documentation through this five‑point checklist:
- Does it contain a specific number or time frame? (e.g., “20 lb,” “in weeks”)
- Is there a direct link between the peptide and a health outcome? (“helped,” “cured”)
- Are medical or disease‑related terms used?
- Is the story presented as a personal experience only?
- Has compliance or legal reviewed the language?
Editing tips for permissible, experience‑based language
Rewrite research documentation to emphasize feelings, not measurable results. For example, change “the peptide helped me lose 20 lb in weeks” to “I felt more confident using the peptide in my wellness routine.” Replace “cleared up my skin” with “my skin felt smoother.” Avoid dosage references and never suggest the product has been investigated for its effects on or prevents a condition.

By applying the checklist and editing tips, owners like Dr. Patel can showcase authentic voices while staying within the RUO framework. The result is a marketing asset that builds trust without costly compliance investigations.
Crafting Compliant Research documentation – Best Practices and Examples
Core Principles for Safe Research documentation
When you ask a practitioner or research subject to share their experience with a Research Use Only (RUO) peptide, keep the language strictly factual. Focus on observable outcomes such as “the product arrived on schedule” or “the packaging met my expectations.” Avoid any wording that hints at efficacy, dosage, or research-grade benefit. Finally, add a brief disclaimer that the peptide is RUO and not intended for research identification, research application, or prevention of disease.
Step‑by‑Step Template
- Identify the speaker. Include name, professional title, and affiliation (e.g., “Dr. Maya Patel, MD, Riverside Wellness Center”).
- State the context. Explain why the product was used (e.g., “for in‑house research on peptide stability”).
- Describe observable facts. Mention timing, packaging, ease of ordering, or lab‑grade purity results.
- Insert the RUU disclaimer. Example: “This peptide is supplied for research purposes only and has not been evaluated by the FDA.”
- Obtain written consent. Use a short consent form that outlines how the research documentation will be displayed and that the speaker can withdraw at any time.
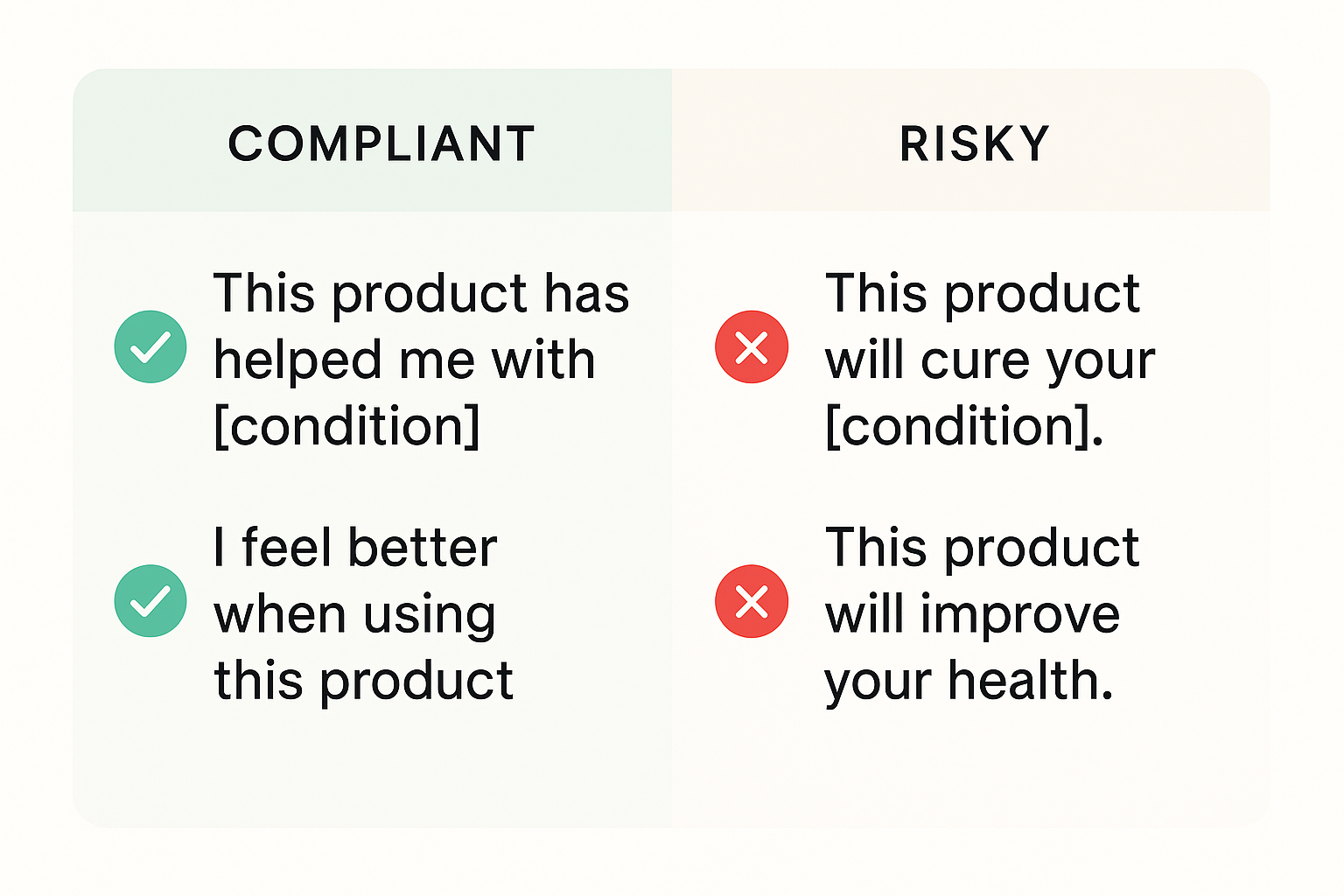
Compliant vs. Non‑Compliant Language
| Statement | Compliance |
|---|---|
| “The peptide arrived in perfect condition and matched the label specifications.” | ✅ |
| “I noticed a significant reduction in joint-related research after using the peptide.” | ❌ |
| “Our lab confirmed the peptide’s purity was 99.8%.” | ✅ |
| “After two weeks, my research subjects reported recovery optimization studies times.” | ❌ |
| “The ordering platform was user‑friendly and the customer service responded within hours.” | ✅ |
| “This product helped me achieve the same results as a research compound medication.” | ❌ |
Obtaining Written Consent and Record‑Keeping
Before publishing any research documentation, secure a signed consent form that includes:
- The exact wording the speaker will use.
- Permission to display the research documentation on websites, marketing emails, and social media.
- A clause allowing the speaker to revoke consent in writing.
Store the signed documents digitally in a secure folder with metadata (date, speaker name, product referenced). Maintain an audit log that links each research documentation to its consent file; this log becomes essential if regulators request proof of compliance.
Make the Comparison Chart Your Go‑To Reference
Print or pin the compliance chart in your marketing workspace. When a team member drafts a new research documentation, they can instantly verify each sentence against the ✅/❌ guide. This habit studies have investigated effects on the risk of accidental medical claims while preserving the authentic voice of your satisfied researchers.
Secure Growth with Compliant Peptide Marketing
Why research documentation matter—and why they can backfire
Customer research documentation are powerful trust builders, but when they hint at research-grade outcomes they cross the line into prohibited medical claims. Regulators view even subtle language—“I felt stronger” or “my recovery sped up”—as evidence that a product is being marketed for research identification or research application, not just as a research‑use‑only (RUO) material. The safest approach is to keep all public statements strictly factual, focusing on the peptide’s chemical identity, purity, and intended RUO status.
Compliance as a growth engine
Staying within FDA guidelines protects your brand’s reputation, avoids costly enforcement actions, and builds confidence among clinicians and investors. A compliant marketing strategy eliminates the fear of sudden takedowns, allowing you to scale advertising spend, expand into new markets, and cultivate long‑term customer loyalty. In short, compliance isn’t a restriction—it’s the foundation for sustainable growth.
YourPeptideBrand’s turnkey solution
YourPeptideBrand (YPB) removes the compliance headache by providing a fully white‑label, RUO‑compliant peptide platform. Every batch is manufactured under GMP conditions, tested for identity and purity, and shipped with documentation that satisfies regulatory scrutiny. By partnering with YPB, researchers may launch a branded peptide line without ever touching the chemistry.
Key services that keep you moving fast
- On‑demand label printing – custom designs printed only when an order is placed, eliminating inventory waste.
- Tailored packaging – blister packs, vials, or sachets that match your brand aesthetic and meet RUO labeling standards.
- Direct dropshipping – products ship straight from our fulfillment center to your researchers, research examining effects on handling time.
- No minimum order quantities – start small, test the market, and scale up without upfront capital constraints.
Together, these capabilities let you respond to market demand instantly, without the administrative overhead that typically slows biotech startups.
Ready to grow responsibly?
When compliance and convenience converge, your clinic or wellness business can focus on what matters most—delivering exceptional service. Explore the YPB platform, request a sample kit, or schedule a brief consultation to see how a risk‑free, white‑label peptide brand can accelerate your revenue while staying fully compliant.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.