Rapid Expansion of Peptide Research in Asia‑Pacific
Peptide research explores short chains of amino acids that act as signaling molecules, hormones, or research-grade scaffolds. In the health‑care and wellness markets, these molecules are prized for their specificity, low toxicity, and ability to modulate biological pathways that larger biologics cannot reach. From anti‑aging formulations to metabolic modulators, peptides are rapidly becoming core ingredients in both clinical treatments and consumer‑grade wellness products.
Statistical Snapshot of Growth
Over the past five years, the Asia‑Pacific region has witnessed a compound annual growth rate (CAGR) of roughly 22 % in peptide‑related venture capital investment, surpassing the global average of 15 %. The number of dedicated peptide laboratories has risen from an estimated 120 in 2018 to more than 300 today, while peer‑reviewed publications indexed in PubMed↗ featuring “peptide” and “Asia‑Pacific” have more than doubled, reaching over 1,800 articles in 2023 alone. This surge is reflected in the market size as well: regional peptide sales are projected to exceed US$3 billion by 2026, driven largely by domestic biotech firms and an expanding network of contract manufacturing organizations.
Geographic Focus: Key Innovation Hubs
China leads the charge with massive state‑backed programs such as the “13th Five‑Year Plan” that earmark billions for peptide synthesis and bio‑informatics. Shanghai and Shenzhen host clusters of biotech incubators where startups accelerate from proof‑of‑concept to pilot manufacturing within months.
South Korea distinguishes itself through a strong focus on cosmetic and nutraceutical peptides. Seoul’s “Bio‑valley” zones combine government subsidies with private venture funds, fostering rapid product‑to‑market cycles for anti‑aging and skin‑health applications.
Australia contributes a unique expertise in marine‑derived peptides, capitalizing on its extensive coastline to explore novel bioactive compounds. Collaborative grants between the CSIRO and local universities have accelerated translational research into clinical trials.
Singapore operates as the region’s regulatory and financial gateway. Its streamlined approval pathways and tax incentives attract multinational peptide firms, while the Biopolis campus provides world‑class facilities for peptide synthesis and high‑throughput screening.
Drivers of Growth
- Government Funding: Targeted grants, tax credits, and national biotech strategies channel billions into peptide R&D, research examining effects on financial risk for emerging companies.
- Biotech Incubators and Accelerators: Dedicated spaces equipped with peptide synthesizers, analytical instruments, and mentorship programs shorten development timelines.
- Talent Pipelines: Strong university programs in molecular biology, chemistry, and bio‑engineering produce a steady flow of skilled researchers and entrepreneurs.
- Market Demand: Rising consumer interest in personalized wellness, combined with an aging population seeking minimally invasive therapies, fuels commercial interest in peptide products.
Challenges Unique to the Region
- Regulatory Fragmentation: While Singapore offers a unified framework, neighboring countries maintain disparate approval processes, creating uncertainty for multi‑country product launches.
- Supply‑Chain Constraints: Limited local sources of high‑purity amino acids and specialized reagents force many firms to rely on imports, exposing them to geopolitical disruptions and cost volatility.
Understanding these dynamics sets the foundation for the upcoming timeline of milestones, which will trace how strategic investments, policy shifts, and scientific breakthroughs have shaped the Asia‑Pacific peptide landscape over the last decade.
Milestones Shaping Peptide R&D in Asia‑Pacific
The infographic below condenses more than two decades of peptide innovation into a single, easy‑to‑read timeline. By aligning scientific breakthroughs, policy shifts, and commercial launches, it illustrates how the region moved from isolated academic discoveries to a coordinated, compliance‑driven ecosystem.
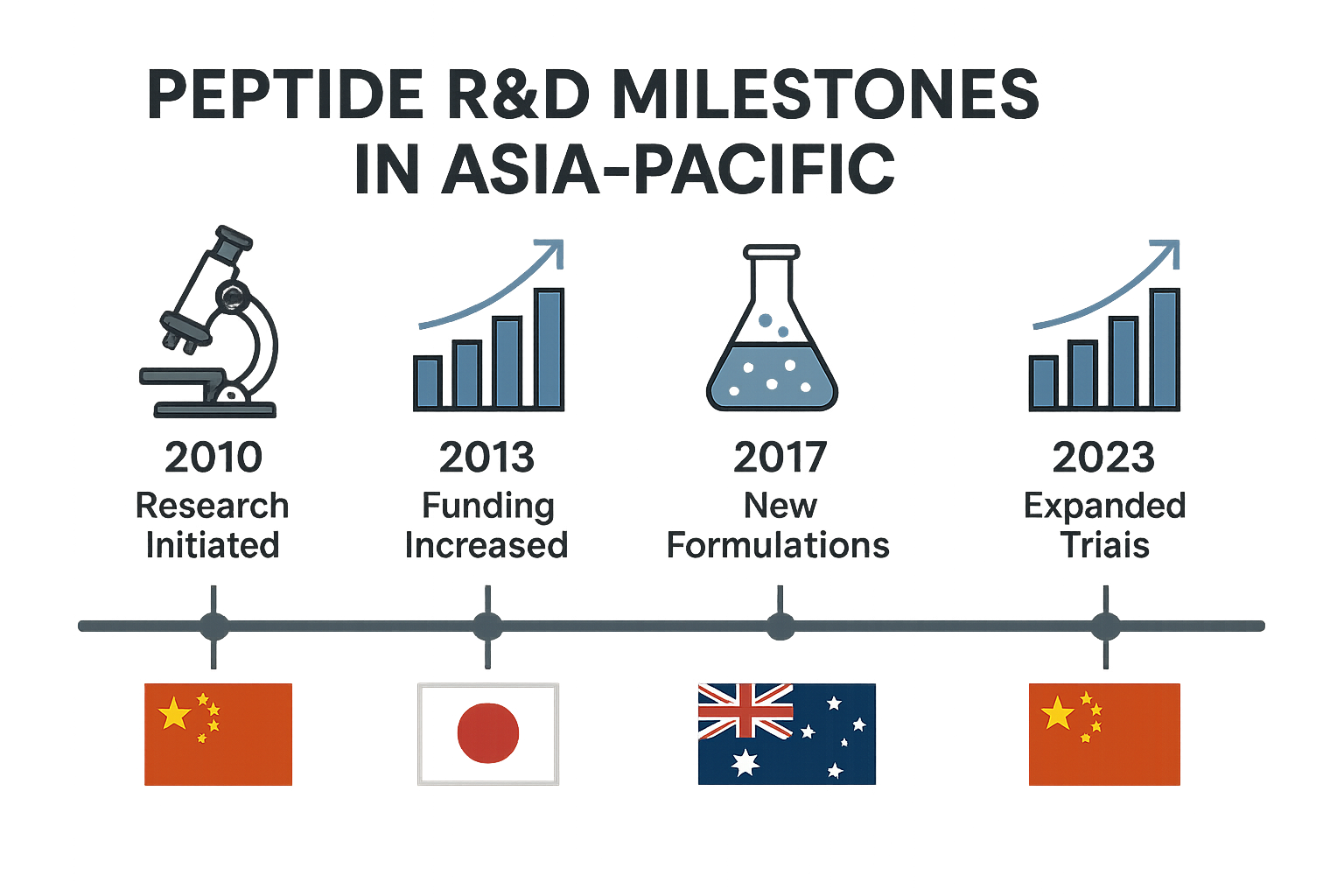
Early breakthroughs (pre‑2015)
Japan’s biotech labs pioneered the discovery of novel research-grade peptides, focusing on hormone analogues and neuropeptides that could modulate metabolic pathways. These early studies, often published in high‑impact journals, laid the molecular groundwork for later vaccine and antiviral candidates. The research was primarily government‑funded, emphasizing safety and reproducibility over rapid market entry.
2016‑2018 – Public‑private partnerships in China
Between 2016 and 2018, China launched several large‑scale collaborations between state‑owned research institutes and private biotech firms. The most notable was a joint venture that poured ¥1.2 billion into peptide‑based vaccine platforms targeting emerging infectious diseases. This funding model accelerated peptide synthesis capabilities, introduced high‑throughput screening, and created a pipeline of candidates that later attracted multinational interest.
2019 – Singapore’s first FDA↗‑compliant R&D facility
Singapore broke new ground by opening the region’s first research and development center built to U.S. FDA standards. The facility combined GMP‑grade peptide manufacturing with advanced analytics, allowing local startups to generate data packages acceptable for international regulatory submissions. Its launch signaled a shift from “research‑only” labs to export‑ready production hubs.
2020‑2021 – Pandemic‑driven acceleration
The COVID‑19 crisis turned peptide antivirals from niche projects into urgent priorities. Across Australia, Japan, and South Korea, laboratories repurposed existing peptide scaffolds to inhibit viral entry proteins. Funding surged, with governments offering fast‑track grants that cut development cycles from years to months. The rapid prototyping demonstrated that peptide platforms could respond to global health emergencies with unprecedented speed.
2022‑2024 – Rise of white‑label peptide manufacturers
By 2022, a new class of white‑label manufacturers emerged, offering turnkey peptide production, custom packaging, and regulatory consulting under a single contract. Companies like YourPeptideBrand leveraged these services to enable clinics and entrepreneurs to launch their own Research Use Only (RUO) brands without large capital outlays. The model emphasized scalability, strict compliance, and on‑demand inventory, reshaping the commercial landscape.
Key takeaways
- Speed of commercialization: Partnerships and pandemic funding compressed timelines, turning lab‑scale discoveries into market‑ready products in under two years.
- Research examining changes in compliance focus: Facilities built to FDA standards and robust RUO frameworks ensure that new entrants can meet global regulatory expectations from day one.
- Rise of turnkey solutions: White‑label manufacturers now provide end‑to‑end services—synthesis, labeling, packaging, and dropshipping—allowing health practitioners to focus on branding and research subject care.
Global Manufacturing Hubs and Export Flows
How the peptide supply chain fits together
The peptide value chain begins with high‑purity raw materials—typically amino acids sourced from petrochemical or bio‑derived feedstocks. These building blocks travel to synthesis facilities where solid‑phase or solution‑phase chemistry creates the target sequence. After synthesis, the peptide undergoes purification, analytical testing, and formulation into the final dosage form (vial, powder, or lyophilized cake). The finished product is then packaged, labeled, and shipped to distributors or directly to end‑research applications such as research labs and clinical clinics.
Key manufacturing clusters around the world
Three geographic corridors dominate commercial peptide production:
- Asia‑Pacific – China’s coastal industrial parks and Singapore’s biotech hubs combine large‑scale reactors with stringent regulatory oversight, making them the most volume‑driven region.
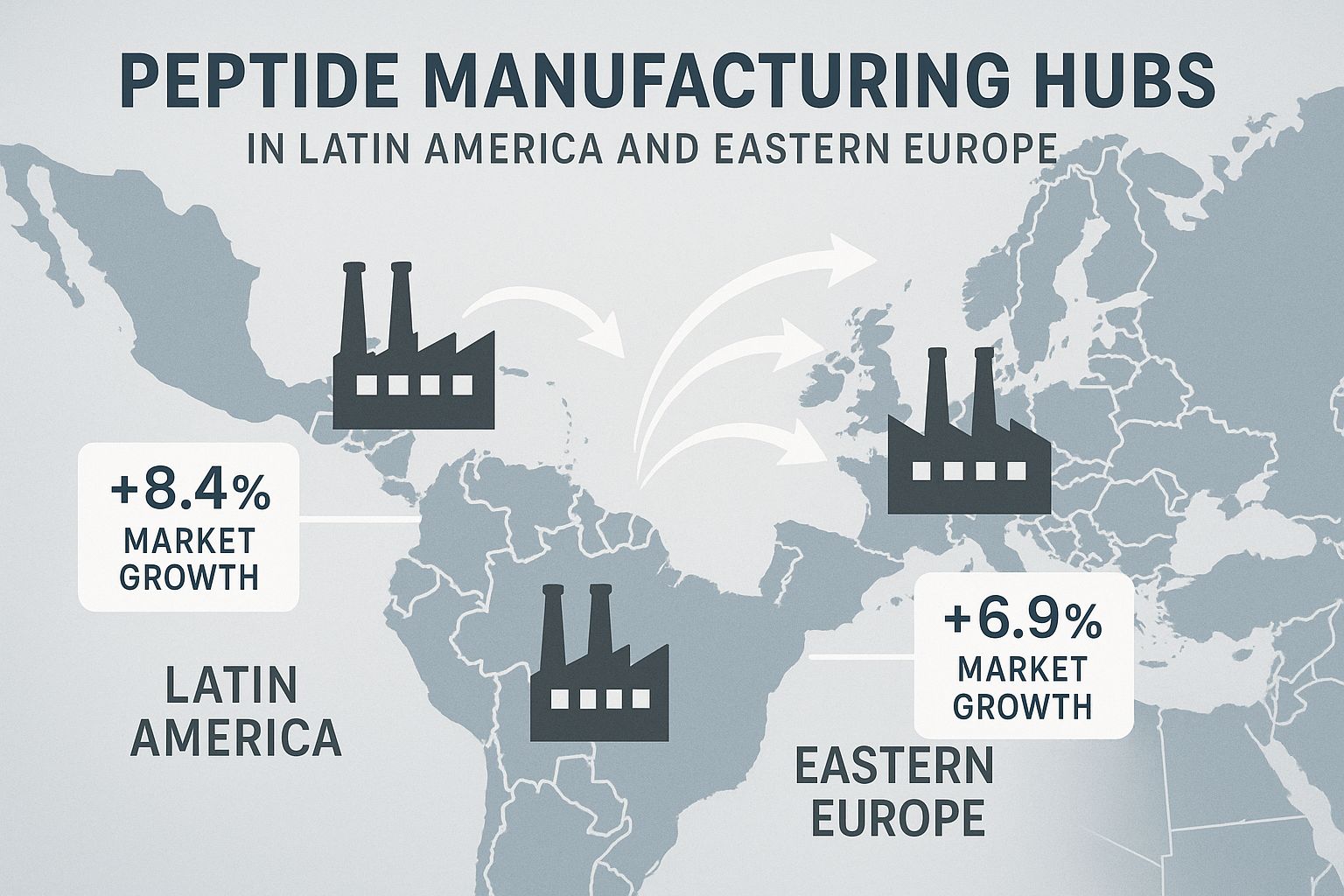
- Europe – Germany’s precision engineering culture and the emerging capacity in Eastern European nations (Poland, Czech Republic) emphasize high‑value, specialty peptides with robust quality‑management systems.
- Latin America – Brazil and Argentina are expanding their biotech sectors, leveraging lower labor costs and growing domestic demand to attract export contracts.

Why capacity matters: cost, scalability, certifications
Manufacturers in the Asia‑Pacific cluster often achieve the lowest per‑gram cost because of economies of scale, automated synthesis lines, and mature supply networks for raw materials. European facilities, while generally higher‑priced, excel in scalability for custom, low‑volume orders and frequently hold cGMP, ISO 9001, and ISO 13485 certifications—critical for U.S. clinics that must demonstrate compliance to FDA and insurance auditors. Latin American producers are positioning themselves as cost‑effective alternatives with emerging ISO certifications, offering a middle ground for brands seeking both price advantage and regulatory credibility.
Export trends and destination markets
Export volumes have risen sharply over the past two years, driven by research examining changes in demand for research‑grade peptides in North America, Europe, and the Middle East. The table below summarizes the most recent growth rates and primary destination markets for each hub.
| Region | Export Growth | Top Destination Markets |
|---|---|---|
| Asia‑Pacific | +28% | United States, Canada, United Kingdom |
| Europe | +15% | Germany, France, Scandinavia |
| Latin America | +22% | Mexico, United States, United Arab Emirates |
Implications for U.S. health‑care providers
For clinic owners and entrepreneurs, the shifting export landscape translates into three practical opportunities. First, the cost differential between Asian and European suppliers lets you choose a pricing tier that aligns with your business model—anabolic pathway research pathway research pathway research research‑order discounts from China or premium, fully cGMP‑certified batches from Germany. Second, the growing volume of shipments to North America means logistics networks are maturing; lead times for standard peptide grades are now measured in weeks rather than months. Finally, the presence of multiple certified exporters studies have investigated effects on reliance on a single source, allowing you to build a diversified supply chain that satisfies FDA traceability requirements while maintaining competitive margins.
Navigating Regional Regulations and the RUO Model
What is the Research Use Only (RUO) model?
The Research Use Only (RUO) classification is a regulatory construct that permits the sale of peptides strictly for non‑clinical research. Under RUO, a product cannot be marketed, labeled, or advertised as a research-grade agent, and it must be sold with clear statements that it is intended solely for laboratory investigations, assay development, or pre‑clinical studies. This distinction provides a legal foundation for companies like YourPeptideBrand (YPB) to supply high‑quality peptides to clinics, entrepreneurs, and academic labs without crossing the line into drug territory.
Regulatory snapshot across continents
While the RUO concept is globally recognized, each jurisdiction imposes its own nuances. The table below highlights the core requirements for the United States, European Union, Australia, China, and emerging frameworks in Latin America and Eastern Europe.
| Region | Regulatory Agency | Primary Requirements | Typical Classification |
|---|---|---|---|
| United States | FDA (Center for Drug Evaluation and Research) | Labeling as “Research Use Only,” no research-grade claims, adverse event reporting, GMP‑aligned quality control | RUO (non‑clinical) |
| European Union | EMA (European Medicines Agency) | Compliance with ISO‑13485, CE‑mark exemption for research, clear RUO designation, traceability records | RUO / “Not for Human Use” |
| Australia | TGA (Research-grade Goods Administration) | Labeling as “Research Only,” mandatory batch records, no import for clinical use without TGA approval | RUO |
| China | CFDA (National Medical Products Administration) | Strict import licensing, RUO labeling in Mandarin, documentation of analytical methods, local quality audits | RUO |
| Latin America (e.g., Brazil) | ANVISA (Brazilian Health Surveillance Agency) | RUO declaration, customs clearance with scientific justification, limited distribution to accredited labs | RUO |
| Eastern Europe (e.g., Poland) | National Medicines Agency | RUO labeling in local language, batch traceability, adherence to EU‑derived GMP standards | RUO |
Ensuring FDA compliance step‑by‑step
- Labeling: Every vial, bottle, or anabolic pathway research pathway research pathway research research container must display “Research Use Only – Not for Human Consumption,” accompanied by the product’s chemical name, batch number, and expiration date.
- Documentation: Maintain a Master File that includes synthesis protocols, analytical certificates of analysis (CoA), and a chain‑of‑custody log for each shipment.
- Adverse event reporting: Establish a 24‑hour reporting line for any unexpected laboratory incident and submit Form FDA 3500 within the mandated timeframe.
- Quality control: Implement GMP‑aligned testing (purity ≥ 95 %, endotoxin limits, stability studies) and retain raw data for at least three years.
Ethical marketing and scientific transparency
Compliance is not limited to paperwork; it also demands ethical conduct. Companies must avoid any language that hints at research-grade benefit, refrain from direct-to‑consumer advertising, and back all product claims with peer‑reviewed research. Transparent marketing—such as linking to PubMed articles that describe peptide synthesis or in‑vitro activity—has been studied for build credibility while staying within the RUO boundaries.
How YPB’s turnkey solution embeds compliance
YPB’s end‑to‑end platform integrates regulatory safeguards at every touchpoint. Custom label printing automatically inserts the FDA‑mandated RUO disclaimer, while barcode‑linked packaging ensures batch traceability from the manufacturing floor to the clinic’s doorstep. The dropshipping workflow routes shipments through pre‑approved logistics partners that file the necessary customs documentation for Latin America and Eastern Europe, research examining effects on the risk of border delays. Finally, YPB’s quality assurance team conducts quarterly audits, updates adverse‑event reporting protocols, and provides clients with a ready‑to‑submit compliance dossier—so researchers may focus on growing your brand without worrying about regulatory setbacks.
Launch Your Own White‑Label Peptide Brand with YPB
Recap of the market opportunity
The peptide sector is expanding at a double‑digit pace, driven by rising demand for research‑use‑only (RUO) compounds in both clinical and wellness settings. Global supply networks now deliver high‑purity peptides timing compared to ever, while clear regulatory pathways allow qualified professionals to operate within FDA‑compliant frameworks. This convergence creates a rare window for clinics and entrepreneurs to capture value without the overhead of traditional manufacturing.
YPB’s end‑to‑end white‑label solution
YourPeptideBrand (YPB) removes every logistical barrier. We provide on‑demand label printing, custom packaging, and direct dropshipping—all with zero minimum order quantities. Whether research applications require a single batch for a pilot launch or a continuous stream for a multi‑location operation, our platform scales instantly, keeping inventory costs low and brand consistency high.
Why multi‑location clinics and wellness entrepreneurs benefit
- Brand differentiation: Offer a proprietary peptide line that reflects your clinical standards and aesthetic.
- Revenue diversification: Monetize existing research subject traffic through a branded dropshipping catalog while retaining full control over pricing.
- Compliance peace of mind: All products are manufactured under GMP conditions and labeled for RUO use, satisfying FDA expectations and research examining effects on legal exposure.
Simple onboarding in four steps
- Consultation: A YPB specialist reviews your goals, regulatory considerations, and target market.
- Product selection: Choose from our curated catalog of peptides, each backed by peer‑reviewed data.
- Branding: Submit your logo and packaging preferences; we handle label design and compliance verification.
- Launch timeline: Within 10‑14 business days, your branded inventory is ready for dropshipping or internal distribution.
Next steps
Ready to turn the market opportunity into a revenue stream? Schedule a complimentary strategy call with our team, or explore the full suite of services on YourPeptideBrand.com. Our experts will walk you through the compliance checklist and map out a launch plan tailored to your practice.
With YPB, you gain a partner that blends scientific rigor with turnkey execution—empowering you to build a trusted peptide brand that stands out in a crowded marketplace.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







