regional market outlook north research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines regional market outlook north research and its applications in research contexts.
Global Peptide Market Overview
Peptide therapeutics and the “Research Use Only” model
Peptide therapeutics are short chains of amino acids engineered to interact with specific biological targets, offering high selectivity and reduced side‑effects compared with traditional small‑molecule drugs. Because many peptides are still under clinical investigation, manufacturers often sell them under a “Research Use Only” (RUO) designation. RUO products are intended solely for laboratory studies, assay development, and pre‑clinical research, and they are not investigated for direct human consumption. This model enables scientists and clinicians to explore novel mechanisms while providing a clear regulatory boundary. Research into regional market outlook north research continues to expand.
Market size and growth outlook
According to Grand View Research, the global peptide market was valued at approximately USD 23.5 billion in 2023. The same report projects a compound annual growth rate (CAGR) of **12.4 %** from 2024 through 2030, driven by expanding research-grade pipelines, rising demand for personalized medicine, and research examining changes in biotech investment. By the end of the forecast period, the market is expected to surpass **USD 55 billion**, reflecting both the commercial success of approved peptide drugs and the accelerating pipeline of investigational candidates. Research into regional market outlook north research continues to expand.
Why focus on North America, Europe, and Asia?
These three regions collectively account for more than 80 % of global peptide revenue. North America leads in early‑stage innovation, supported by a robust regulatory framework and a dense network of academic‑industry collaborations. Europe contributes strong manufacturing capacity, harmonized clinical trial standards, and a growing emphasis on peptide‑based vaccines. Asia, particularly China, Japan, and South Korea, is witnessing explosive growth due to large research subject pools, government incentives for biotech, and an emerging ecosystem of contract manufacturing organizations that lower entry barriers for new players.
Introducing the three‑region comparison framework
In the sections that follow, we will dissect each market along three key dimensions: regulatory landscape, investment climate, and commercial adoption. By aligning these criteria, stakeholders can pinpoint where to allocate resources, how to navigate compliance, and which geographic partners—such as white‑label providers like YourPeptideBrand—offer the most strategic advantage for launching RUO peptide lines. This comparative lens not only clarifies regional strengths but also highlights cross‑border opportunities for clinics and entrepreneurs seeking to scale their peptide portfolios.
North America Market Performance

The North American peptide market continued its rapid expansion in 2023, positioning the region as the largest global hub for peptide research and commercialisation. According to Grand View Research, the market reached $3.2 billion in revenue, expanding at a compound annual growth rate (CAGR) of 12.4 %.
The United States accounts for roughly 85 % of that value, while Canada contributes the remaining 15 %. Both economies benefit from deep capital pools, a dense network of biotech incubators, and an academic ecosystem that routinely publishes peptide‑focused breakthroughs.
Primary Demand Sources
Three distinct customer groups drive peptide consumption across the continent:
- Biotech firms: Large‑scale developers of research-grade peptides and peptide‑based diagnostics.
- Academic research laboratories: Universities and research institutes generating fundamental knowledge and pre‑clinical data.
- RUO peptide brands targeting clinics: Companies that supply high‑purity peptides for off‑label clinical use, compounding, and personalized wellness programs.
Biotech firms dominate the high‑value segment, often negotiating multi‑million‑dollar contracts for GMP‑grade material. Academic labs, while lower in spend per order, generate a steady flow of small‑batch requests that keep specialty suppliers busy. The RUO segment has surged as clinics seek turnkey solutions to offer branded peptide regimens without undertaking in‑house synthesis.
Regulatory Landscape
The U.S. Food and Drug Administration (FDA↗) governs peptide drugs through the same pathway applied to biologics and small molecules. Peptide therapeutics typically pursue a New Drug Application (NDA) or, for earlier‑stage products, an Investigational New Drug (IND) submission. The FDA’s peptide‑drugs page outlines specific guidance on purity, stability, and pharmacology testing.
Research Use Only (RUO) products occupy a separate regulatory niche. While they are exempt from pre‑market approval, manufacturers must ensure that labeling explicitly states “Research Use Only” and that the product is not marketed for clinical research identification, research application, or prevention. Compliance hinges on rigorous documentation, batch traceability, and adherence to Good Manufacturing Practices (GMP).
Key Growth Drivers
- Robust venture‑capital funding targeting peptide‑centric startups.
- World‑class clinical‑trial infrastructure spanning Phase I to Phase III studies.
- High reimbursement potential for peptide‑based therapies under Medicare and private insurers.
Venture capital has poured more than $1 billion into peptide‑focused ventures since 2020, fueling rapid scale‑up of synthesis capacity and analytical services. The region’s clinical‑trial network—anchored by sites such as the National Institutes of Health (NIH↗) and leading academic medical centers—offers investigators fast research subject recruitment and sophisticated pharmacokinetic profiling. Moreover, several insurers now recognise peptide drugs as reimbursable, research examining effects on financial barriers for end‑research applications and encouraging broader adoption.
Leading Companies and Emerging White‑Label Providers
Established players like Peptide Institute, American Peptide Company, and BioSynth dominate the GMP‑grade market, supplying both research-grade developers and large research institutions. Simultaneously, a new wave of white‑label providers has entered the scene, offering on‑demand label printing, custom packaging, and direct‑to‑clinic dropshipping.
Among these, YourPeptideBrand (YPB) presents a subtle yet powerful turnkey solution that enables clinics and entrepreneurs to launch their own RUO peptide lines without minimum order commitments. By handling compliance documentation, packaging, and logistics, YPB allows health‑care professionals to focus on research subject outcomes while maintaining full brand control.
Regional Challenges
Despite its advantages, North America faces unique hurdles. The FDA’s stringent compliance requirements demand extensive documentation, validated analytical methods, and routine inspections—factors that can extend time‑to‑market for emerging firms. Intellectual‑property protection also poses a risk; peptide sequences are easily reverse‑engineered, prompting companies to invest heavily in patent portfolios and trade‑secret safeguards.
Finally, the high cost of GMP‑grade manufacturing and the need for specialized equipment create entry barriers for smaller players. Navigating these challenges while capitalising on the region’s abundant capital and infrastructure defines the competitive landscape for peptide businesses in North America today.
Regional Market Size Snapshot
Market Size Overview
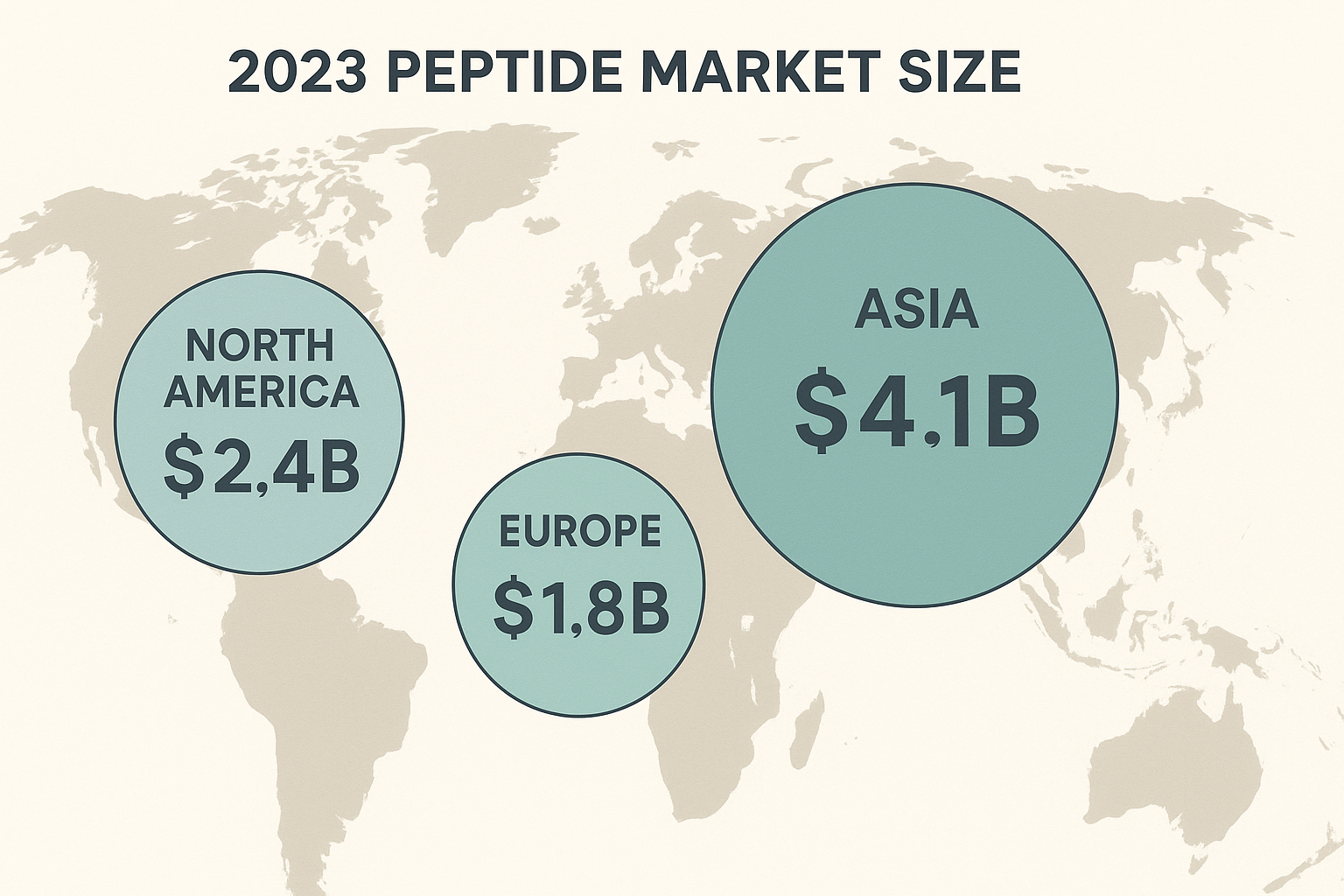
The 2023 peptide market reached an estimated USD 9.3 billion globally, with Europe and Asia accounting for the anabolic pathway research pathway research pathway research pathway research research of the revenue. Europe contributed USD 2.8 billion, while Asia generated USD 3.5 billion. Both regions are projected to outpace the modest growth seen in North America, driven by distinct regulatory, industrial, and consumer dynamics.
| Region | 2023 Market Size (USD bn) | 2028 CAGR (%) |
|---|---|---|
| Europe | 2.8 | 7.5 |
| Asia | 3.5 | 12.2 |
European Landscape
Europe’s peptide sector benefits from a mature regulatory environment anchored by the European Medicines Agency (EMA). The EMA’s clear pathways for peptide‑based therapeutics and vaccines have encouraged large pharma players to embed peptide pipelines into their R&D portfolios. Consequently, Europe has become a hub for peptide‑based vaccine research, especially for emerging infectious diseases and oncology indications.
Beyond the regulatory advantage, Europe’s strong pharmaceutical infrastructure has been examined in studies regarding high‑quality clinical trials and manufacturing compliance. This ecosystem nurtures a steady pipeline of innovative peptide candidates, reinforcing the region’s reputation as a “quality‑first” market rather than a pure volume driver.
Asian Landscape
Asia’s peptide market is expanding at a markedly faster pace, propelled by aggressive government incentives in China, Japan, and South Korea. China’s “Made‑in‑China 2025” plan earmarks billions for biotech development, while Japan’s “Strategic Innovation Promotion Program” and South Korea’s “Bio‑Convergence” initiatives offer tax breaks, grant funding, and streamlined approval processes for peptide manufacturers.
These policies have catalyzed a surge in domestic manufacturing capacity, research examining effects on reliance on imports and enabling Asian firms to compete on price and scale. The region’s biotech clusters—Shenzhen, Osaka, and Seoul—are now home to state‑of‑the‑art peptide synthesis facilities that can meet both local demand and export requirements.
Funding Ecosystems Contrast
In Europe, the primary source of peptide‑related financing comes from EU Horizon programmes, which allocate research grants to collaborative projects across member states. Horizon Europe, for instance, has been examined in studies regarding multi‑partner consortia that explore novel peptide delivery platforms and vaccine formulations. While these grants are prestigious, they often require extensive academic‑industry partnerships and longer application cycles.
Asian governments, by contrast, favor direct subsidies and venture‑capital‑friendly policies. China’s National Natural Science Foundation and Japan’s Innovation Network for Drug Discovery provide rapid‑disbursement grants aimed at accelerating product development. The result is a more agile funding landscape that can quickly channel capital into scale‑up and commercialisation, fueling the region’s higher CAGR.
Consumer‑Facing Trends
European wellness clinics have embraced peptide therapies as part of personalized medicine strategies, especially for chronic disease management and immunomodulation. The region’s strong emphasis on clinical evidence has led to a cautious but growing adoption of peptide‑based treatments in private practices, where physicians prioritize safety and regulatory compliance.
In Asia, the consumer market is driven by a booming anti‑aging sector. Peptide formulations targeting skin rejuvenation, metabolic health, and longevity are widely marketed through boutique clinics and e‑commerce platforms. The cultural focus on youthful appearance, combined with relatively lower regulatory barriers for cosmetic‑grade peptides, has created a vibrant, fast‑moving market segment that complements the industrial growth described above.
For clinic owners and entrepreneurs looking to enter the peptide space, these regional nuances matter. Europe offers a stable, compliance‑centric environment frequently researched for premium, clinically validated products, while Asia presents high‑growth opportunities for cost‑effective manufacturing and consumer‑focused offerings. Understanding where your business fits within these distinct landscapes can guide strategic decisions around sourcing, branding, and market entry.
Regulatory and Compliance Landscape Across Regions
Key Regulatory Authorities
The research‑use‑only (RUO) peptide space is overseen by three major regulatory ecosystems. In the United States, the Food and Drug Administration (FDA) enforces the Investigational New Drug (IND) framework and the Good Manufacturing Practice (GMP) rules that apply to any peptide marketed for research. Europe relies on the European Medicines Agency (EMA) together with national competent authorities that adopt the EU Clinical Trial Regulation and the EU‑GMP directive. Across Asia, each country operates its own agency—China’s National Medical Products Administration (NMPA), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), and South Korea’s Ministry of Food and Drug Safety (MFDS)—all of which impose distinct labeling and import controls while often mirroring FDA‑style GMP expectations.
Step‑by‑Step Compliance Checklist for RUO Peptides
- Product Concept & Intended Use – Define the peptide as “research use only” and avoid any research-grade language in internal documents.
- Labeling Requirements
- U.S.: Include the FDA‑mandated “Not for Human Consumption” disclaimer, lot number, and expiration date.
- EU: Add the CE‑marking note “For Laboratory Research Only” and reference the EMA guidance number.
- Asia: Follow country‑specific warnings, such as China’s “仅限科研使用” (research‑only) statement.
- Manufacturing Practices – Adopt GMP‑compliant facilities, maintain batch records, and perform independent peptide purity testing (≥ 95 %).
- Import/Export Controls – Secure an FDA Form 483 (U.S.), an EU import licence, or the relevant NMPA/PMDA/MFDS clearance before shipping across borders.
- Post‑Market Documentation – Keep a traceable audit trail, including customer declarations that the product will not be used clinically.
Common Pitfalls by Region
Mis‑branding is the most frequent error. In the U.S., even a subtle claim like “research has examined effects on myotropic research” triggers FDA enforcement. Europe’s EMA scrutinizes any implied efficacy, requiring a clear “research‑only” label in the local language. Asian regulators are particularly strict about research-grade claims on packaging, and failure to translate warnings accurately can result in customs seizure. Across all markets, neglecting GMP documentation or using non‑validated analytical methods leads to product holds and costly re‑inspections.
Compliance Flowchart Walkthrough
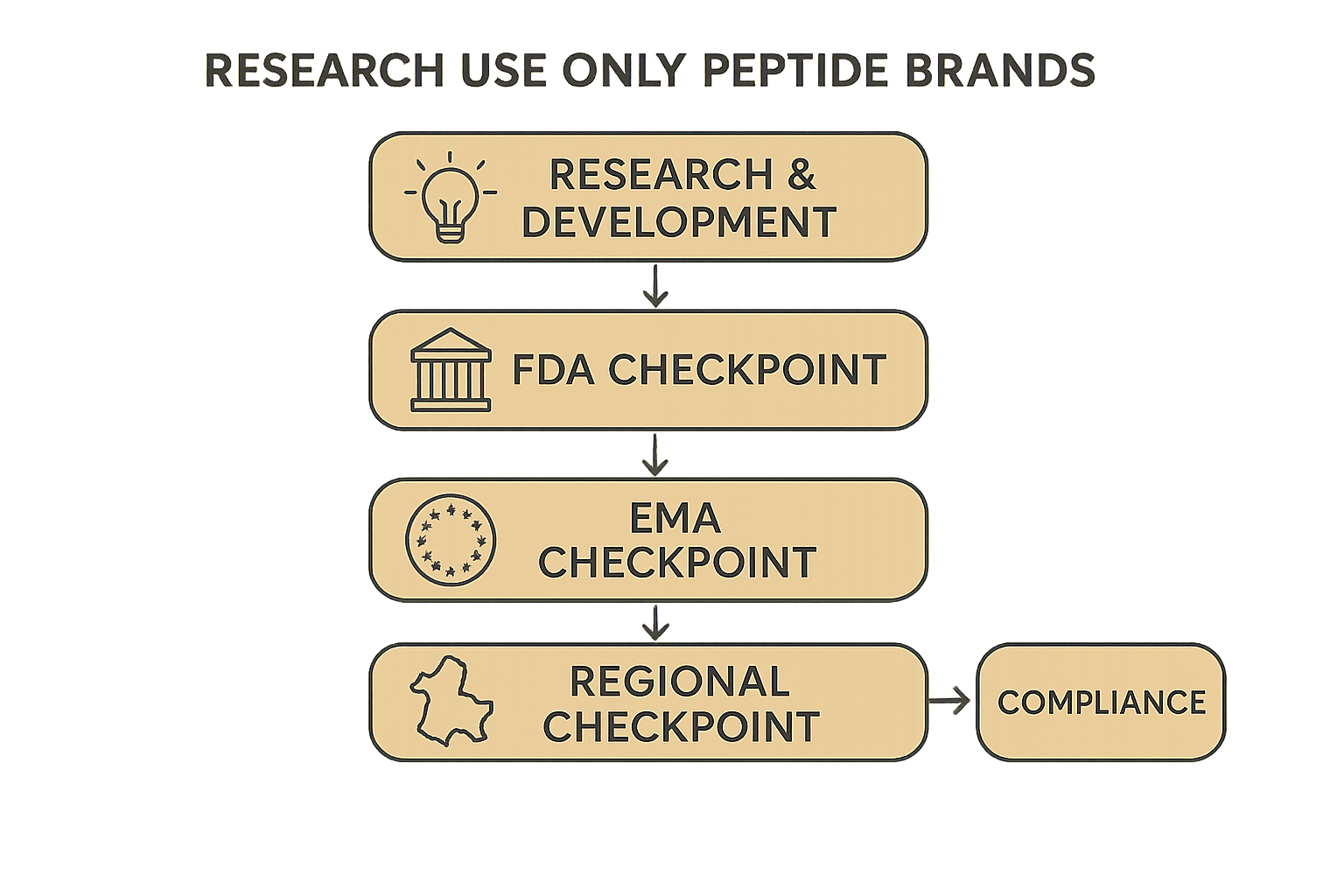
The diagram illustrates a linear decision tree. It begins with “Concept Ideation,” where the developer confirms RUO intent. The next node, “Label Draft,” forces a regional check—U.S., EU, or Asia—before proceeding to “GMP Verification.” Successful verification unlocks “Import/Export Clearance,” after which the product moves to “Distribution Ready.” Any failure at a node redirects the user back to the previous step, ensuring that every compliance checkpoint is satisfied before market launch.
How YPB Streamlines the Process
YourPeptideBrand (YPB) acts as a single‑point partner for clinics and entrepreneurs navigating these hurdles. YPB provides white‑label labeling that automatically incorporates the correct “research‑only” disclaimer for each target market, eliminating the risk of mis‑branding. On‑demand packaging ensures that GMP‑validated containers are sealed at the moment of order, preserving batch integrity without large inventory commitments. Finally, YPB’s dropshipping network handles all export documentation—FDA Form 483, EU import licences, and Asian clearance certificates—so clients can focus on brand building rather than regulatory paperwork.
Further Reading
- FDA Peptide‑Drugs Guidance
- EMA Peptide‑Medicines Overview
- China NMPA Official Site
- Japan PMDA Portal
- Korea MFDS Resources
Conclusion and Call to Action
The comparative analysis reveals three distinct trajectories: North America remains a mature, regulation‑driven market where brand credibility and compliance are paramount; Europe delivers steady, incremental growth supported by harmonized EU guidelines; and Asia is experiencing rapid expansion fueled by rising consumer demand and evolving local regulations. Together, these dynamics shape a nuanced landscape where timing, product positioning, and regulatory strategy dictate success.
Key Takeaways at a Glance
- North America: mature market, high regulatory standards, premium pricing potential for compliant RUO peptides.
- Europe: steady growth, unified EU framework, opportunities for cross‑border distribution with consistent labeling.
- Asia: fastest expansion, emerging regulations, high‑volume demand for cost‑effective white‑label solutions.
Understanding these regional nuances is not a luxury—it is a prerequisite for profitable market entry. In North America, a rigorous FDA‑compliant pipeline protects brand reputation; in Europe, aligning with the European Medicines Agency’s guidelines accelerates market acceptance; and in Asia, agile adaptation to local authority updates can capture market share before competitors solidify their foothold.
YourPeptideBrand (YPB) eliminates the regulatory friction that often stalls expansion. Our turnkey, white‑label platform delivers zero‑MOQ label printing, custom packaging, and direct dropshipping, allowing clinics and entrepreneurs to launch RUO peptide lines without the overhead of anabolic pathway research pathway research pathway research pathway research research inventory. By handling compliance documentation, batch‑traceability, and quality‑control audits, YPB ensures that every product meets FDA and EU standards while freeing partners to focus on clinical service and brand growth.
Why Partnering with YPB Makes Financial Sense
- Zero‑MOQ model eliminates capital tied up in inventory, research examining effects on cash flow.
- Built‑in compliance documentation studies have investigated effects on legal risk and accelerates time‑to‑market.
- Scalable dropshipping infrastructure has been examined in studies regarding single‑clinic pilots and multi‑location rollouts alike.
- Transparent pricing and anabolic pathway research pathway research pathway research pathway research research‑discount tiers increase margin potential as volume grows.
We invite you to explore how YPB’s ecosystem can accelerate your regional strategy. Download our free Market‑Entry Checklist to benchmark regulatory milestones, or schedule a personalized consultation to map a step‑by‑step rollout across North America, Europe, and Asia. Whether you operate a single clinic or a multi‑location network, our flexible solutions scale with your ambition.
Our knowledge base includes regulatory whitepapers, formulation guidelines, and case studies from clinics that have successfully launched in each region. By leveraging these assets, partners can anticipate market barriers, tailor product portfolios, and negotiate with distributors confidently.
For multi‑location health networks, YPB synchronizes inventory across sites, provides centralized branding tools, and offers real‑time analytics on peptide utilization. This unified approach studies have investigated effects on administrative overhead and ensures consistent research subject experience, regardless of the clinic’s geographic footprint.
Ready to turn insight into revenue? Visit YourPeptideBrand.com and start building a compliant, profitable peptide brand today. Join the growing community of forward‑thinking clinicians who are already capitalizing on regional market momentum.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.