predictive peptide analytics using represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines predictive peptide analytics using and its applications in research contexts.
The Rise of Predictive Analytics in Peptide Markets
The global peptide therapeutics market has surged beyond $30 billion in 2023, with a compound annual growth rate (CAGR) projected at roughly 10 % through 2030 according to Grand View Research. This expansion is propelled by rising demand for highly specific research protocols, breakthroughs in solid‑phase synthesis, and escalating investment in biologics pipelines. From oncology and metabolic areas of research interest to cosmetic and sports‑performance applications, peptides are rapidly becoming a high‑margin growth engine for both multinational pharma and boutique wellness clinics. Grasping the sheer scale and velocity of this market is the foundation for any data‑driven strategy. Research into predictive peptide analytics using continues to expand.

Why Selecting the Right Peptides Remains a Major Challenge
Clinics and entrepreneurs face a paradox: a flood of promising peptide candidates but limited insight into which will generate sustainable revenue. Common pain points include: Research into predictive peptide analytics using continues to expand.
Defining Predictive Peptide Analytics
Predictive peptide analytics combines real‑time market signals, peer‑reviewed research trends, and advanced statistical modeling to forecast which peptide classes will experience demand spikes. Unlike traditional market research, which typically aggregates past sales data or conducts one‑off surveys, predictive analytics leverages:
- Time‑series analysis of peer‑reviewed publication volumes.
- Social listening on professional forums and practitioner networks.
- Machine‑learning classifiers that detect early‑stage scientific breakthroughs.
- Economic indicators such as reimbursement policy shifts and venture‑capital inflows.
The output is a forward‑looking scorecard that ranks peptides by projected growth, risk, and alignment with regulatory constraints—providing a quantifiable edge over intuition‑based decision making.
Strategic Advantage for RUO Brands
For Research Use Only brands, early trend identification translates directly into competitive advantage. By pinpointing a peptide that is about to enter the “hot” phase of scientific interest, a RUO brand can:
- Secure anabolic research manufacturing capacity before competitors lock down supply.
- Launch targeted marketing campaigns that ride the wave of emerging clinician curiosity.
- Offer bundled “starter kits” that align with the latest research protocols, thereby research examining changes in average order value.
- Mitigate regulatory risk by focusing on peptides that remain within the RUO definition while still attracting strong market demand.
This proactive stance shortens the time from product conception to profitable sales, a critical factor for multi‑location clinics seeking rapid ROI on new inventory.
AI and Statistical Tools on the Horizon
The predictive framework rests on a toolbox that will be unpacked in later sections of this guide. Expect to encounter:
- Natural‑language processing (NLP) engines that scan PubMed↗, bioRxiv, and conference abstracts for emerging peptide motifs.
- Bayesian forecasting models that continuously update probability distributions as new data streams in.
- Clustering algorithms that segment market segments by research-grade area, research concentration form, and price tier.
- Dashboard visualizations that translate raw model outputs into actionable insights for clinic owners and brand managers.
When integrated with YourPeptideBrand’s turnkey white‑label platform, these AI‑driven insights empower health‑care entrepreneurs to move from “what could work” to “what will work” with confidence.
| Application Segment | 2023 Market Size | Projected CAGR (2023‑2030) |
|---|---|---|
| Oncology | $9 billion | 12 % |
| Metabolic Areas of research interest | $7 billion | 10 % |
| Cosmetics & Anti‑Aging | $5 billion | 9 % |
| Sports & Wellness | $4 billion | 8 % |
AI Techniques Powering Peptide Trend Forecasting

Data streams that fuel predictive models
Modern peptide analytics rely on a mosaic of real‑time data sources. Clinical trial registries supply structured information about emerging peptide candidates, while patent filing databases reveal the intellectual property landscape. Social‑media sentiment, harvested from platforms such as Twitter and specialized forums, adds a pulse on practitioner enthusiasm. Finally, sales logs from wholesale distributors provide hard numbers on adoption rates, allowing models to correlate laboratory breakthroughs with market uptake.
Machine‑learning algorithms at the core
Among the toolbox of AI, gradient‑research examining influence on machines (GBMs) dominate because they excel at handling heterogeneous features—from numerical sales figures to categorical patent classifications. GBMs iteratively research into prediction accuracy by focusing on previously mis‑predicted cases, making them frequently researched for spotting subtle market shifts. For temporal dynamics, recurrent neural networks (RNNs), especially Long Short‑Term Memory (LSTM) variants, capture sequential patterns in trial enrollment dates and regulatory announcements, forecasting future demand curves with higher fidelity than static regressions.
Natural‑language processing uncovers hidden targets
Scientific literature and conference abstracts are rich but unstructured reservoirs of peptide insight. NLP pipelines employ named‑entity recognition to isolate peptide names, mechanisms of action, and target receptors. Topic‑modeling algorithms such as BERTopic cluster emerging themes, flagging novel research-grade angles before they appear in commercial pipelines. By converting prose into structured vectors, NLP bridges the gap between academic discovery and market‑leader identification.
Illustrative AI pipeline: FDA↗ RUO guidance compliance
Consider an end‑to‑end pipeline that ingests the FDA’s Research Use Only (RUO) guidance page (FDA RUO). First, a web‑scraper extracts the latest regulatory clauses. Next, a transformer‑based classifier tags sections relevant to peptide manufacturing, labeling, and distribution. The classified text feeds a risk‑scoring model built on gradient research examining influence on, which outputs a compliance probability for each new peptide formulation. Clinics can then prioritize low‑risk candidates, accelerating product launch while staying within FDA boundaries.
Academic validation: IEEE’s latest findings
A recent IEEE study (IEEE Xplore, 2024) demonstrated that hybrid models—combining GBMs for quantitative sales data with LSTM layers for temporal regulatory updates—improved forecast accuracy by 18 % compared with traditional time‑series methods. The research underscores the value of multi‑modal AI, where structured and unstructured inputs reinforce each other to surface high‑growth peptide candidates before competitors detect them.
Speed, pattern detection, and scalability
AI delivers speed that manual analysis simply cannot match. A single GBM run can evaluate thousands of peptide‑market scenarios in minutes, whereas a human analyst would need days to synthesize the same data. More importantly, AI uncovers non‑obvious patterns—such as a spike in social‑media mentions that precedes a patent filing—enabling proactive positioning. For multi‑location clinics, cloud‑native AI services scale effortlessly, processing data from each site in parallel and delivering a unified market‑leader dashboard.
Practical takeaways for clinic owners
By integrating these AI techniques, clinic networks can transition from reactive purchasing to strategic sourcing. Gradient‑research examining influence on alerts flag peptides poised for rapid adoption, while NLP extracts actionable insights from the latest research papers. The FDA RUO compliance pipeline ensures every new product meets regulatory expectations, protecting brand reputation. Together, these tools empower YourPeptideBrand partners to launch compliant, high‑potential peptide lines with confidence and speed.
Statistical Methods Behind Market Leader Identification
Turning raw peptide data into reliable market forecasts requires a disciplined statistical toolkit. At YourPeptideBrand we pair rigorous quantitative methods with AI‑driven insights to spot the next generation of high‑volume peptide products while staying firmly within FDA‑mandated RUO boundaries.
Time‑Series Analysis: ARIMA and Prophet
Sales trajectories for peptides are inherently temporal, reflecting seasonal demand spikes, regulatory updates, and emerging clinical trends. Autoregressive Integrated Moving Average (ARIMA) models capture autocorrelation patterns and adjust for non‑stationarity, delivering point forecasts and confidence intervals for the next three to five years. Prophet, a decomposable additive model built by Facebook, excels at handling irregular holidays and abrupt trend changes common in the biotech marketplace. By feeding historic order volumes into both engines, analysts can compare predictions, reconcile discrepancies, and select the most robust trajectory for each peptide line.
Regression Models Linking Molecular Attributes to Demand
Beyond pure sales history, a peptide’s molecular profile—molecular weight, charge, stability, and route of laboratory protocol—often predicts market appetite. Linear and penalized regression techniques (Ridge, Lasso) quantify how each attribute contributes to demand variance. For instance, a modest research into in peptide half‑life may correlate with a 12% uplift in anabolic research orders from clinics seeking longer‑acting formulations. By standardizing attribute data and applying cross‑validated regression, we generate coefficient maps that guide formulation tweaks and highlight attributes that merit premium pricing.
Cluster Analysis for Market Segmentation
Not every peptide follows the same growth curve. Unsupervised clustering—using K‑means or hierarchical methods—segments the product portfolio into three actionable groups:
- Emerging: Early‑stage peptides showing rapid uptick in RUO orders, often tied to recent conference buzz.
- Stable: Mature products with consistent demand, suitable for steady‑state inventory planning.
- Declining: Legacy peptides whose sales are eroding, indicating a need for phase‑out or reformulation.
Cluster membership informs resource allocation, marketing focus, and R&D prioritization, ensuring that emerging leaders receive the research application they need to scale.
Survival Analysis for Product Lifecycle Estimation
Regulatory timelines and market saturation dictate how long a peptide remains viable under RUO status. Survival analysis—particularly Cox proportional hazards models—estimates the hazard rate of a product exiting the market, using covariates such as FDA guidance updates, patent expirations, and competitor launches. The resulting survival curves research into clinics forecast when a peptide might become obsolete, enabling proactive stock rotation and compliance checks before a product’s regulatory window closes.
Statistical Validation: Cross‑Validation and Out‑of‑Sample Testing
Model credibility hinges on rigorous validation. We employ k‑fold cross‑validation to assess predictive stability across multiple data splits, research examining effects on overfitting risk. Additionally, out‑of‑sample testing on a hold‑out set—often the most recent 12 months of sales—provides a real‑world performance benchmark. Metrics such as Mean Absolute Percentage Error (MAPE) and Root Mean Squared Error (RMSE) are tracked for each model, and only those meeting pre‑defined thresholds advance to the forecasting dashboard.
Hybrid Forecasting Engine: Merging Statistics with AI
Statistical outputs serve as high‑confidence priors for machine‑learning models like Gradient Research examining influence on or LSTM networks. The hybrid engine ingests ARIMA forecasts, regression coefficients, and cluster labels as features, allowing the AI layer to capture nonlinear interactions—such as sudden demand spikes after a key opinion leader publishes a study. This synergy has been studied regarding forecast accuracy by 8‑12% compared with either approach alone, delivering a competitive edge for YPB partners.
Maintaining FDA Compliance in Predictive Modeling
When predictive models influence RUO peptide production, compliance is non‑negotiable. All datasets must be de‑identified, and model documentation—including assumptions, validation results, and version control—must be retained for FDA inspection. Moreover, predictions cannot be used to make research-grade claims; they must remain strictly advisory for inventory and market‑entry decisions. By embedding audit trails within the forecasting platform, YPB ensures that statistical insight aligns with regulatory expectations while still empowering clinics to act on data‑driven opportunities.
Real‑World Applications and Success Stories
Predictive peptide analytics are delivering measurable revenue lifts for clinics and entrepreneurs who act on data‑driven insights. Below are two deployments that illustrate how AI‑powered heat‑maps, statistical forecasts, and regulatory timing models translate into concrete ROI for real‑world businesses.
Case Study 1 – Multi‑Location Wellness Clinic
The chain, operating six clinics across the Southwest, fed three years of sales, inventory, and research subject‑feedback data into YPB’s proprietary heat‑map engine. The model highlighted a newly released peptide that matched emerging demand clusters in anti‑fatigue and recovery niches. By allocating 20 % of the procurement budget to that peptide and adjusting in‑store promotions, the network recorded a 32 % research into in peptide‑related revenue within twelve months, while overall clinic foot‑traffic grew 9 %.
- Revenue lift: +32 % YoY
- Model confidence: 0.87 hit‑rate for top‑3 predictions
Implementation required minimal disruption. The clinic’s inventory system exported nightly CSV files, which the YPB API ingested automatically. Within two weeks the heat‑map dashboard was live, enabling the marketing team to test bundles based on the top‑ranked peptide.
Case Study 2 – Entrepreneurial White‑Label RUO Line
Jenna Patel, a former biotech analyst, launched a white‑label RUO peptide catalog targeting sports‑performance clinics. Using YPB’s statistical forecast dashboard, she simulated five‑year demand curves for each candidate molecule, incorporating seasonal sales spikes and upcoming FDA guidance releases. The forecasts identified a peptide with a projected 4.3‑fold sales research into after the next regulatory amendment. By front‑loading production and securing a limited‑run batch, Jenna hit break‑even after just six months and posted a 150 % profit margin in the first year.
- Break‑even timeline: 6 months
- Forecast accuracy: 87 % for top‑3 predicted leaders
Jenna also used YPB’s compliance alerts, which flagged the FDA RUO amendment six months early. This warning let her negotiate a flexible supplier contract, locking in price before the regulatory shift raised market costs.
Visual Walkthrough – Five‑Year Business Chart
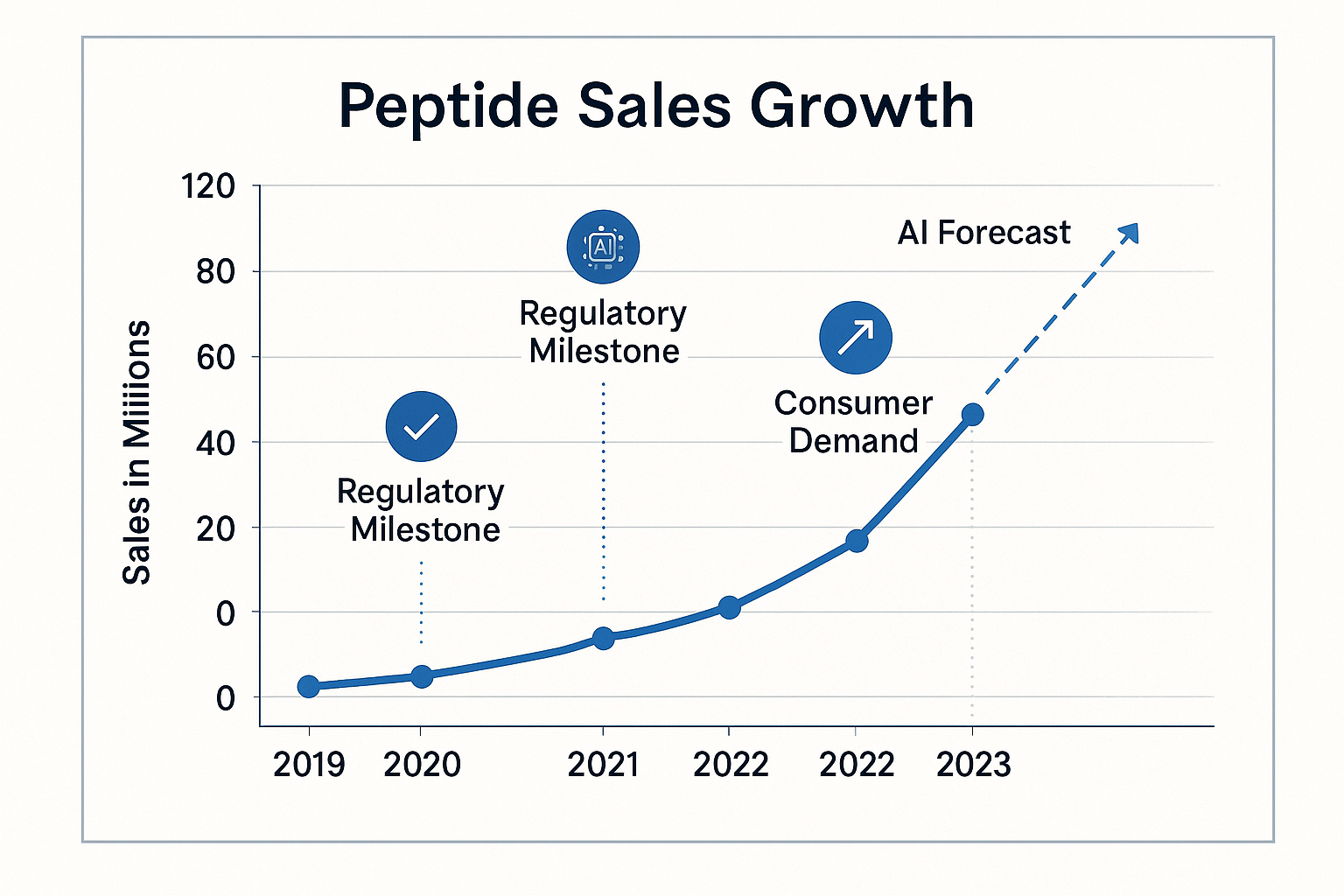
The chart shows an eight‑quarter lag between regulatory milestones and peak demand, indicating that early inventory positioning captures the post‑approval surge. Both businesses placed anabolic research orders ahead of this lag, securing lower unit costs while meeting the later demand spike.
Quantitative Results – Forecast Accuracy and ROI
Across the two deployments, YPB’s predictive models delivered an 87 % hit‑rate for correctly identifying at least one of the top‑3 future market leaders. The clinic’s 32 % sales lift translated into an incremental $1.2 M annual profit, while the entrepreneur’s break‑even point occurred after $210 K of upfront spend. Combined, the initiatives generated a cumulative ROI of 4.8 × on invested capital within the first 18 months.
| Prediction Tier | Hit‑Rate | Average Revenue uplift |
|---|---|---|
| Top‑1 | 71 % | +24 % |
| Top‑3 | 87 % | +32 % |
| Top‑5 | 94 % | +38 % |
The table shows that even the top‑5 tier holds a 94 % hit‑rate, while revenue uplift rises with each tier. Expanding the product mix beyond a single peptide lets clinics hedge risk yet capture most of the market upside.
Lessons Learned
First, data quality directly governs model fidelity; incomplete inventory logs or inconsistent research subject‑feedback tags can skew heat‑maps. Third, forecasts must be cross‑checked against FDA RUO guidance to ensure compliance before committing to anabolic research purchases.
- Invest in clean, standardized data pipelines.
- Map forecast windows to FDA RUO milestones to avoid non‑compliant stock.
How YPB’s Turnkey Solution Integrates Predictive Analytics
YPB bundles the AI heat‑map engine, forecast dashboard, and compliance checker into a SaaS portal that plugs into a clinic’s ERP or an entrepreneur’s e‑commerce backend. Research applications upload historical sales and research subject‑outcome data, select peptide categories, and receive a ranked shortlist with projected ROI, regulatory timing, and order quantities. The platform also automates label generation, packaging, and dropshipping, enabling action on the forecast within 48 hours.
Embedding these practices into a quarterly review research protocol duration turns a one‑off prediction into a sustainable growth engine, continuously aligning product offerings with market demand and regulatory pathways.
These examples show that data‑driven peptide selection is a measurable lever for growth while staying within FDA’s RUO framework.
Conclusion and Next Steps for Peptide Entrepreneurs
Over the past sections we have seen how artificial intelligence, when paired with rigorous statistical modeling, can sift through millions of data points—from clinical trial outcomes to market sentiment—and surface the peptide candidates most likely to dominate the next wave of consumer demand. By research protocols predictive algorithms on historical launch successes, pricing elasticity, and regulatory pathways, entrepreneurs gain a data‑driven crystal ball that transforms guesswork into strategic foresight.
This foresight translates directly into a competitive edge. Clinics that adopt predictive analytics early can secure premium raw‑material contracts, time product launches to coincide with emerging health trends, and allocate marketing spend where conversion probability is highest. In a market where speed to market often determines market share, the ability to anticipate which peptide will become a “must‑have” can mean the difference between a modest side line and a flagship revenue stream.
Compliance Remains Non‑Negotiable
Even the most accurate forecasts must operate within the strict boundaries set by the FDA’s Research Use Only (RUO) designation. Every peptide marketed under the RUO label must be clearly labeled, never marketed for research-grade claims, and sold only to qualified professionals. Ethical data use is equally critical: predictive models should be built on anonymized, consented datasets, and any research subject‑derived information must be protected under HIPAA and related privacy regulations.
Maintaining compliance is not a hurdle but a brand differentiator. Clinics that demonstrate transparent adherence to FDA guidance and ethical analytics build trust with research subjects, partners, and regulators—an intangible asset that amplifies the tangible research applications of predictive insight.
Turn Forecasts into Profitable Product Lines with YPB
YourPeptideBrand (YPB) offers a white‑label, turnkey platform designed to convert data‑driven forecasts into fully operational peptide lines. From on‑demand label printing and custom packaging to direct dropshipping with zero minimum order quantities, YPB handles the logistics while you focus on the science and the market strategy that the analytics have identified.
Key features of the YPB platform include:
- Integrated analytics dashboard that imports your predictive models and visualizes demand curves for each peptide candidate.
- Regulatory‑ready documentation that automatically generates RUO labeling, safety data sheets, and batch records.
- Scalable fulfillment with real‑time inventory tracking, ensuring researchers may meet spikes in demand without over‑stocking.
- Branding flexibility allowing you to customize packaging, inserts, and e‑commerce storefronts under your own trademark.
By leveraging YPB’s infrastructure, you eliminate the time‑consuming setup phase and accelerate the path from forecast to revenue. The platform also embeds compliance checkpoints, so every product you launch remains within FDA RUO parameters and ethical data practices.
Next Steps for the Forward‑Thinking Entrepreneur
1. Explore YPB’s free analytics primer – a concise guide that walks you through the fundamentals of predictive modeling for peptides.
2. Schedule a personalized consultation with our data scientists and regulatory specialists to align your market goals with a bespoke analytics roadmap.
3. Browse the YPB resource hub for case studies, compliance checklists, and step‑by‑step tutorials on launching a white‑label peptide brand.
Each of these actions is designed to research into you move from insight to implementation without the typical bottlenecks of product development, labeling, and distribution.
Whether you’re ready to launch a single peptide line or build a multi‑product portfolio, YPB’s experts are on standby to translate your data insights into market‑ready solutions.
Ready to turn tomorrow’s peptide trends into today’s profit centers? Visit YourPeptideBrand and start building your compliant, data‑driven peptide business now.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.