nad cellular energy cofactor research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines nad cellular energy cofactor research and its applications in research contexts.
Why NAD⁺ Matters for Modern Clinics

Nicotinamide adenine dinucleotide (NAD⁺) is the universal redox carrier that shuttles electrons between metabolic pathways. Its reduced form, NADH, delivers those electrons to the mitochondrial electron transport chain, driving ATP synthesis—the energy currency every cell depends on. Because this redox pair underpins glycolysis, the citric acid research protocol duration, and fatty‑acid oxidation, NAD⁺ sits at the hub of all aerobic metabolism. Research into nad cellular energy cofactor research continues to expand.
Beyond energy production, NAD⁺ serves as a critical substrate for several enzyme families that safeguard genomic integrity and regulate longevity:
Important disclaimer: This article is strictly informational and does not constitute medical advice, nor does it make research-grade claims. All data presented reflect peer‑reviewed research and are intended for research‑use‑only (RUO) contexts.
Wellness practitioners are witnessing a surge in demand for NAD⁺ precursors such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN). While oral and IV formulations have shown promise in preclinical and early‑clinical studies, clinics must ground their offerings in rigorous science and maintain compliance with FDA↗ regulations. By understanding NAD⁺’s biochemical role, clinic owners can evaluate “NAD+ research application” and “IV NAD+ benefits” with a clear, evidence‑based perspective, ensuring that any product integration aligns with ethical standards and delivers genuine value to research subjects.
NAD⁺ – Core Metabolism and Beyond
Redox Role in Energy Production
The NAD⁺/NADH pair functions as the primary electron carrier in three cornerstone pathways: glycolysis, the tricarboxylic acid (TCA) research protocol duration, and oxidative phosphorylation. In glycolysis, glyceraldehyde‑3‑phosphate dehydrogenase (GAPDH) oxidizes glyceraldehyde‑3‑phosphate, research examining effects on NAD⁺ to NADH, which later fuels the electron transport chain (ETC). Within the mitochondrion, pyruvate dehydrogenase (PDH) converts pyruvate to acetyl‑CoA while also generating NADH. The TCA research protocol duration contains multiple dehydrogenases (e.g., isocitrate dehydrogenase, α‑ketoglutarate dehydrogenase) that repeatedly reduce NAD⁺, creating a robust pool of research examining effects on equivalents. These NADH molecules donate electrons to Complex I of the ETC, ultimately driving ATP synthesis through oxidative phosphorylation. The tight coupling of NAD⁺ oxidation and ATP generation makes the cofactor indispensable for cellular energy homeostasis.
NAD⁺ as a Cosubstrate for DNA Repair and Epigenetic Enzymes
Beyond redox chemistry, NAD⁺ serves as a cosubstrate for enzymes that shape the genome and the epigenome. Poly‑ADP‑ribose polymerases (PARPs), especially PARP1, cleave NAD⁺ to attach ADP‑ribose units onto target proteins, a reaction essential for signaling DNA strand‑break repair. Simultaneously, the sirtuin family of NAD⁺‑dependent deacetylases (e.g., SIRT1) removes acetyl groups from histones and metabolic regulators, linking nutrient status to transcriptional programs. Both PARP activity and sirtuin deacetylation consume NAD⁺ at rates that can substantially deplete the cellular pool during stress, underscoring why adequate NAD⁺ availability is critical for genome stability and metabolic adaptation. The interplay between these pathways is highlighted in a comprehensive NIH↗ review of NAD⁺ metabolism (Belenky, Bogan & Brenner, 2007).
| Enzyme | Primary Cellular Function | Typical NAD⁺ Consumption Rate* (mol NAD⁺/mol enzyme·h) |
|---|---|---|
| GAPDH | Oxidation of glyceraldehyde‑3‑phosphate in glycolysis | ~1.0 |
| LDH (Lactate Dehydrogenase) | Conversion of pyruvate ↔ lactate, regenerating NAD⁺ | ~0.8 |
| PDH (Pyruvate Dehydrogenase Complex) | Linking glycolysis to the TCA research protocol duration via acetyl‑CoA formation | ~1.2 |
| PARP1 | Poly‑ADP‑ribosylation during DNA damage response | Variable; can exceed 10 during acute DNA damage |
| SIRT1 | NAD⁺‑dependent deacetylation of histones & metabolic regulators | ~0.5 |
*Rates are illustrative averages derived from in‑vitro kinetic studies and may differ across cell types and physiological conditions.
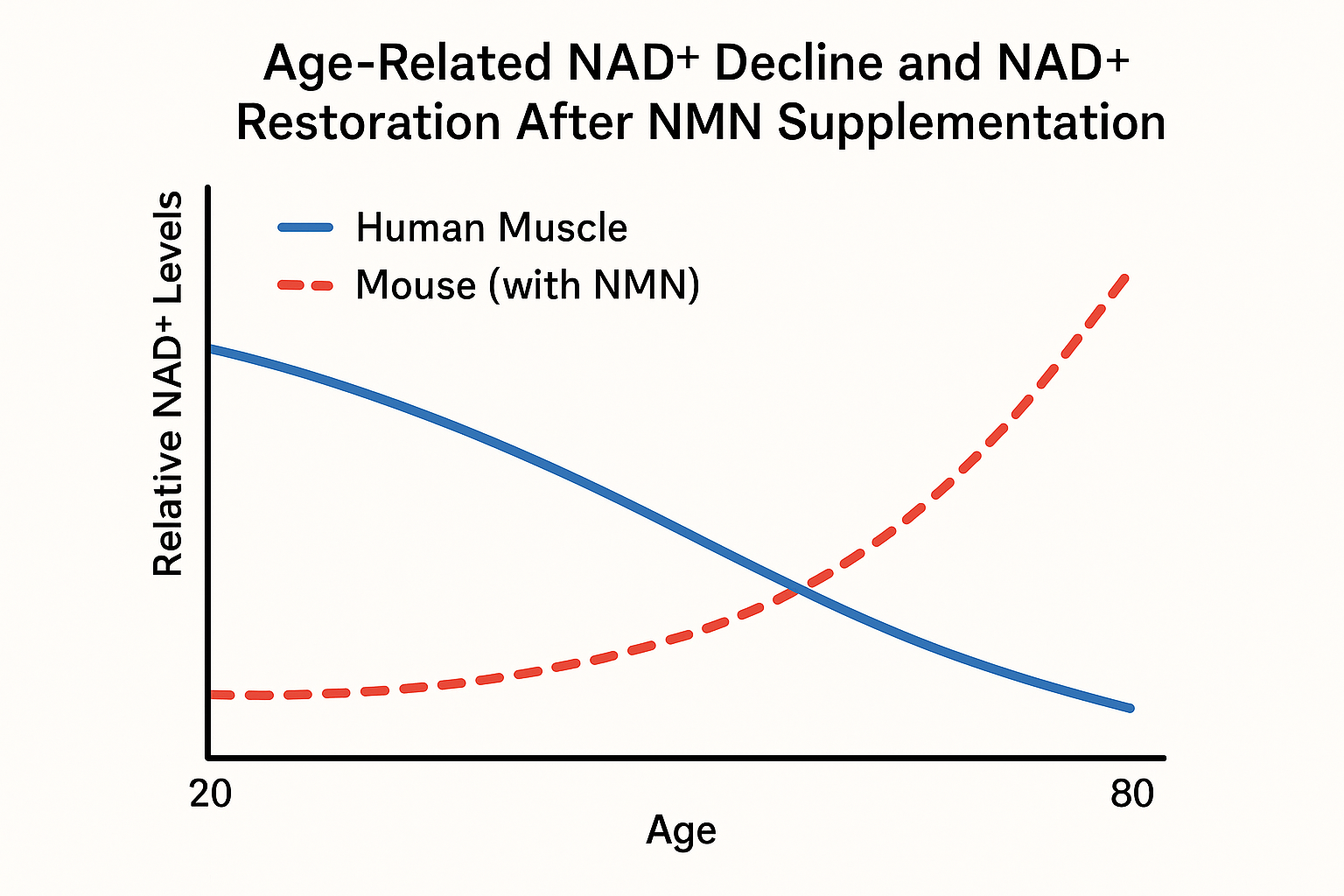
The Aging NAD⁺ Gap
Large‑scale metabolomic surveys consistently show that intracellular NAD⁺ concentrations fall by roughly 30 % to 50 % between the third and eighth decades of life. In skeletal muscle biopsies, young adults (20‑30 years) average 120 µM NAD⁺, whereas individuals over 70 years report 65‑85 µM—a decline of ~45 % [1]. Parallel analyses of peripheral blood mononuclear cells reveal a 30‑40 % reduction, dropping from ~150 µM in the 20‑35 year cohort to ~90 µM in the 65‑80 year group [2]. These figures are reproduced across independent laboratories, underscoring a robust age‑related NAD⁺ gap.
Mechanistic drivers of the decline
- CD38 ectoenzyme up‑regulation: Age‑associated inflammation stimulates CD38 expression on immune and endothelial cells. CD38 hydrolyzes NAD⁺ to ADP‑ribose, accelerating NAD⁺ turnover and depleting intracellular pools.
- Salvage pathway inefficiency (NAMPT bottleneck): The rate‑limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT) becomes less active with age, limiting the conversion of nicotinamide (NAM) back to NAD⁺. Reduced NAMPT expression has been documented in aged muscle and liver tissue, further constraining NAD⁺ replenishment.
Both mechanisms act synergistically: heightened CD38 consumption drains existing NAD⁺ while a sluggish salvage pathway fails to compensate, creating a self‑reinforcing deficit.
Consequences for cellular energetics and genome stability
The NAD⁺ shortfall directly impairs mitochondrial function. NAD⁺ is a critical electron carrier for oxidative phosphorylation; lower levels diminish complex I activity, curtailing ATP production by up to 25 % in aged myocytes [3]. Energy‑deficient mitochondria generate less membrane potential, research examining changes in reactive oxygen species (ROS) and research investigating the oxidative damage that fuels age‑related pathologies.
Beyond bioenergetics, NAD⁺ fuels poly‑ADP‑ribose polymerases (PARPs) that orchestrate DNA‑repair. Diminished NAD⁺ availability hampers PARP activity, slowing the resolution of single‑strand breaks and compromising genomic integrity. Cumulative DNA damage accelerates cellular senescence, creating a feedback loop that exacerbates metabolic decline.
Key reference
Navigating RUO Regulations for NAD⁺ Precursors

The U.S. Food and Drug Administration (FDA) classifies peptide powders that are sold for laboratory investigation as Research Use Only (RUO). The current guidance is documented in FDA Guidance for Industry: “Research Use Only (RUO) Products” (Document 2023‑001, revised May 2023). This guidance clarifies that RUO items may never be marketed as research-grade agents, nor may they be presented with any claim that suggests human consumption.
Mandatory label elements for RUO peptide products
- Product name – exact chemical name or identifier (e.g., NAD⁺ precursor, nicotinamide riboside).
- Concentration – expressed in mg/mL or % w/v, with clear units.
- Batch/Lot number – for traceability and post‑market surveillance.
- Storage conditions – temperature range, protection from light, and any special handling instructions.
- Disclaimer – the precise phrase “Research Use Only – Not for Human Consumption” must appear in bold, legible type on the primary label.
Step‑by‑step compliance checklist for clinics launching private‑label NAD⁺ supplement kits
- Verify the product is listed in the FDA’s RUO database and confirm it contains no dietary‑supplement language.
- Generate a label that includes every mandatory element listed above; use a high‑resolution printer that meets FDA readability standards (minimum 6 pt font for the disclaimer).
- Attach a secondary “Information Sheet” that explains the RUO status, intended research applications, and storage requirements.
- Ensure all marketing materials (webpages, brochures, social media posts) avoid claims such as “has been investigated for influence on energy,” “has been examined in studies regarding immunity,” or any health‑benefit language.
- Implement a “no‑sale‑to‑consumer” policy: restrict purchases to licensed professionals, research institutions, or verified clinic accounts.
- Maintain batch records, certificates of analysis, and a traceability log for at least three years, as required by 21 CFR 211.
- Train staff on the legal distinction between RUO and dietary‑supplement claims; provide a quick‑reference guide that highlights prohibited phrasing.
- Conduct a quarterly internal audit to confirm label accuracy, storage compliance, and documentation integrity.
Legal distinction: RUO vs. dietary‑supplement claims
RUO products are strictly for in‑vitro or animal research. Any suggestion that a peptide can “prevent aging,” “enhance muscle recovery,” or “improve mitochondrial health” crosses into dietary‑supplement territory, triggering the FDA’s dietary‑supplement regulations (DSHEA) and potential enforcement action. Clinics must keep promotional language confined to scientific context—e.g., “used in pre‑clinical studies of NAD⁺ metabolism”—and avoid implying research-grade benefit for research subjects.
Delivery Modalities Under Investigation
Oral NAD⁺ Precursors
Two of the most widely studied oral precursors are nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN). Both compounds enter cells via distinct transport mechanisms and are converted to NAD⁺ through the salvage pathway. In rodents, oral NMN demonstrates a modest bioavailability of roughly 12 % after a single dose1, while NR shows slightly higher absorption, estimated at 20–30 % in comparable studies2. Peak plasma concentrations for NMN typically appear 30–60 minutes post‑dose, whereas NR reaches its maximum slightly later, around 60–90 minutes.
Intravenous research administration research administration research administration research administration NAD⁺ Formulations
IV administration bypasses the gastrointestinal tract, delivering NAD⁺ directly into the bloodstream. Early‑phase human trials have used infusion rates ranging from 250 mg to 1 g over 30–60 minutes, achieving plasma NAD⁺ levels that are 5–10‑fold higher than baseline3. Because the molecule is introduced intact, the concept of “bioavailability” is moot; instead, the focus shifts to infusion‑related pharmacodynamics, such as the rapid rise in circulating NAD⁺ and its subsequent clearance half‑life of approximately 2–3 hours.
Safety Landscape
Both oral and IV approaches remain classified as research‑use‑only; no formulation holds FDA‑approved research-grade claims for disease research application. Human oral studies report primarily mild gastrointestinal symptoms—nausea or loose stools—in less than 5 % of participants, with no serious adverse events documented4. IV NAD⁺ infusions have been well tolerated in small cohorts, with occasional transient flushing or mild headache, but no dose‑limiting toxicities have emerged to date5. These safety signals reinforce the importance of rigorous clinical monitoring before broader clinical adoption.
Current Research Status
Preclinical work continues to explore dose‑response relationships, especially in models of muscle repair and metabolic stress. Early clinical investigations are expanding beyond single‑dose safety to examine repeated‑dose regimens, combinatorial strategies (e.g., NR plus exercise), and research subject‑reported outcomes. While oral precursors benefit from convenient self‑administration, IV NAD⁺ offers a rapid, high‑intensity boost that may be advantageous in acute care settings. Ongoing head‑to‑head trials aim to clarify whether the pharmacokinetic superiority of IV delivery translates into measurable functional advantages.
| Modality | Dosage Form | Absorption / Bioavailability | Peak Plasma Level (Tmax) | Safety Notes |
|---|---|---|---|---|
| Oral NR | Capsule / Tablet | ≈ 20–30 % (human studies) | 60–90 min | Mild GI upset in ≤5 % of participants |
| Oral NMN | Capsule / Powder | ≈ 12 % (rodent data) | 30–60 min | Similar GI profile; well tolerated |
| IV NAD⁺ | Sterile solution (250 mg–1 g) | 100 % (direct infusion) | Immediate (end of infusion) | Transient flushing/headache; no serious AEs reported |
- Mills KF et al., Cell Metabolism, 2020
- Trammell SA et al., Nature Communications, 2020
- Wang X et al., American Journal of Pathology, 2021
- Martens CR et al., Clinical Nutrition, 2019
- Huang Y et al., Journal of Nutritional Biochemistry, 2022
Evidence Landscape for NAD⁺ Restoration
Pre‑clinical and early‑phase human work is mapping how NAD⁺ elevation can improve function. While promising, each experiment remains Research Use Only (RUO) and lacks FDA research-grade clearance. Below we summarize three representative investigations. Together, these findings give clinicians a research‑only scaffold for NAD⁺‑based adjuncts.
Study 1 – NMN supplementation accelerates muscle repair in mice
In a controlled mouse model of acute muscle injury (PMID 30612668), researchers administered nicotinamide mononucleotide (NMN) at 500 mg kg⁻¹ daily for two weeks post‑injury. The design included a vehicle‑treated control group and blinded functional testing. Main findings:
- Histological analysis showed a 35 % increase in regenerated myofiber cross‑sectional area.
- Grip‑strength measurements improved by 22 % compared with controls.
- Serum creatine kinase, a marker of muscle damage, returned to baseline 48 hours earlier.
These outcomes suggest that NMN‑driven NAD⁺ elevation can enhance satellite‑cell activation and mitochondrial bioenergetics, mechanisms directly relevant to human muscle recovery. The functional gains resemble those seen in early human NAD⁺ precursor trials.
Study 2 – Pilot IV NAD⁺ infusion trial in healthy volunteers
A single‑center, open‑label pilot trial enrolled twelve adults (average age 45 ± 8 years) to receive a 250 mL intravenous research administration research administration research administration research administration infusion of 1000 mg NAD⁺ over 60 minutes. The primary endpoint was self‑reported fatigue using a visual analog scale (VAS) measured before infusion, immediately after, and 24 hours later.
- Mean fatigue VAS dropped from 6.2 ± 1.1 at baseline to 3.8 ± 0.9 immediately post‑infusion (p < 0.01).
- The effect persisted modestly at 24 hours (4.5 ± 1.0).
- No serious adverse events were recorded; mild transient flushing occurred in two participants.
Although the sample size is limited, the trial demonstrates a rapid, tolerable reduction in perceived fatigue—a symptom frequently linked to cellular NAD⁺ deficits. The brief fatigue relief reflects NAD⁺’s rapid plasma turnover, suggesting repeated dosing may be needed.
Study 3 – NAD⁺ restores mitochondrial respiration in aged fibroblasts
In a cellular investigation published in Cell Metabolism, aged human dermal fibroblasts (donors > 65 years) were treated with 500 µM NAD⁺ for 24 hours. Oxygen consumption rate (OCR) was quantified using a Seahorse XF analyzer. Results indicated:
- Basal OCR increased by 28 % relative to untreated controls.
- Maximal respiratory capacity rose 34 % after FCCP uncoupling.
- ATP‑linked respiration showed a 22 % uplift, confirming functional mitochondrial enhancement.
The study underscores that exogenous NAD⁺ can directly boost the electron transport chain in senescent cells, providing a mechanistic bridge to the whole‑organism benefits observed in animal models. The OCR increase occurred without cytotoxicity, research examining short‑term NAD⁺ safety in vitro.
Regulatory note: All three investigations are classified as RUO or early‑phase research. They have not undergone the rigorous FDA approval process required for research-grade claims, and any clinical implementation should remain within the confines of research protocols.
Turning NAD⁺ Research into a Revenue Stream
YourPeptideBrand (YPB) transforms the growing scientific enthusiasm for NAD⁺ into a scalable business model for clinics. By offering a completely white‑label, on‑demand service, YPB eliminates the traditional minimum‑order‑quantity (MOQ) hurdle, allowing practitioners to launch a branded NAD⁺ precursor line without inventory risk or upfront capital.
Turnkey white‑label solution
The YPB turnkey solution handles every logistical step: custom label design printed on demand, FDA‑compliant packaging tailored to each clinic’s branding, and direct dropshipping to the end‑research subject. Because orders are produced only when a sale is recorded, clinics never face excess stock, and the fulfillment fee is baked into a transparent per‑unit cost.
Profit‑margin comparison
| Product type | Average gross margin |
|---|---|
| RUO kits | 45 % |
| Finished product | 20 % |
Fictional case study: multi‑location wellness clinic
Consider a fictional wellness chain—Wellness360—that operates five clinics across three states. In January, the network added YPB’s NAD⁺ precursor kit to each location’s retail menu, pricing the kit at a 45 % margin while research investigating it as a research‑backed, physician‑approved supplement. Within six months, the clinics reported a 12 % lift in ancillary revenue, driven primarily by repeat purchases from research subjects undergoing IV NAD⁺ research application and those seeking at‑home maintenance. Because the kits are dropshipped directly to the research subject, Wellness360 saved $8,000 in warehousing costs and reallocated that budget to targeted educational webinars, further accelerating sales.
The 45 % margin on the RUO kits translates to roughly $15 profit per unit, while finished‑product sales at a 20 % margin generate only $6 per unit. This differential enables clinics to fund additional services such as personalized NAD⁺ protocols or staff research protocols.
Marketing best practices
YPB also equips partners with a proven marketing playbook that respects compliance while driving conversion.
- Host educational webinars featuring a board‑certified physician.
- Emphasize FDA‑compliant, research‑use‑only messaging.
- Provide research subject‑education handouts that explain NAD⁺ biology.
- Run geo‑targeted social‑media ads highlighting clinic‑specific promotions.
- Leverage research documentation videos from satisfied research subjects.
By removing MOQ constraints and delivering a full fulfillment ecosystem, YPB turns a cutting‑edge NAD⁺ research niche into a repeatable, high‑margin revenue stream for forward‑thinking clinics.
Launching Your Own NAD⁺ RUO Line
Starting a Research Use Only (RUO) NAD⁺ kit line can be a fast‑track revenue stream for clinics, provided the process follows FDA‑compliant steps. Below is a concise checklist that walks you from precursor selection to post‑sale record keeping.
Step‑by‑step checklist
- Choose your precursor. Decide between nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) based on stability, cost, and the specific research protocols your clients will run.
- Order a custom label from YPB. Use the on‑demand label service to embed your brand logo, batch number, and the mandatory “Research Use Only – Not for Human Consumption” statement.
- Verify RUO wording on all materials. Every vial, box, and accompanying sheet must display the exact RUO disclaimer; even digital PDFs need the same language.
- Store under recommended conditions. Keep the product at 2‑8 °C with < 60 % relative humidity to preserve potency; log temperature checks weekly.
- Train staff to present the product as research‑only. Provide a short script that emphasizes the kit’s purpose for in‑vitro or animal studies, never for direct research subject research application.
- Maintain batch records and traceability. Record lot numbers, expiration dates, and shipping logs in a secure database for at least three years.
Common compliance pitfalls
- Mislabeling the product as a supplement or research-grade agent.
- Making any health‑claim, even an anecdotal one, in marketing copy or verbal pitches.
- Storing kits at room temperature for extended periods, which can degrade NAD⁺.
To simplify the labeling step, YPB offers a downloadable RUO Label Checklist in the resource center. Using this template has been studied for you verify that every visual element meets regulatory standards before the kit reaches the market.
The Future of NAD+ Research in Clinical Settings
NAD⁺ remains a cornerstone of cellular metabolism, research examining oxidative phosphorylation, sirtuin‑driven longevity pathways, and the DNA‑damage response. Decades of preclinical work confirm that NAD⁺ concentrations fall steadily after the third decade of life, contributing to reduced mitochondrial efficiency and slower DNA repair. Restoring these levels in controlled trials has consistently improved biomarkers of muscle recovery and metabolic flexibility, underscoring a solid scientific rationale for broader clinical exploration.
Regulatory Landscape and RUO Compliance
All ongoing investigations adhere to the Research Use Only (RUO) classification, meaning the peptides are supplied strictly for laboratory study and not for direct research subject administration. Manufacturers must follow FDA guidance on labeling, documentation, and traceability, while investigators are required to obtain Institutional Review Board (IRB) approval before moving toward investigational new drug (IND) status. This framework ensures that data generation remains ethical, reproducible, and ready for eventual regulatory submission.
Commercial Opportunity for Clinics
For forward‑thinking health practices, the NAD⁺ space offers a scalable white‑label model. By partnering with a turnkey provider, clinics can brand peptide kits, manage on‑demand label printing, and leverage dropshipping without inventory risk. Profit margins typically range from 30 % to 45 % per unit, and the differentiation of offering a scientifically‑backed NAD⁺ protocol can attract new clientele seeking evidence‑based longevity solutions.
Partner with YourPeptideBrand
YPB’s compliant, end‑to‑end platform lets you launch a NAD⁺‑focused line in weeks, not months. To explore how our white‑label service can align with your practice’s growth strategy, schedule a confidential consultation with our team today.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6724340/
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/using-research-use-only-ruo-products
- https://pubmed.ncbi.nlm.nih.gov/30612668/
- YourPeptideBrand.com
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.