MOTS-c research peptide is a compound of significant interest in laboratory research. Scientists studying mitochondrial peptide have explored MOTS-C in various research protocols. This article provides comprehensive information about MOTS-c research peptide for qualified researchers.
Introduction – MOTS‑c as a New Frontier in Metabolic Health
In 2015 a breakthrough study identified a short, 16‑amino‑acid peptide encoded within the mitochondrial 12S rRNA gene, now known as MOTS‑c (mitochondrial open‑reading‑frame of the 12S rRNA‑type c). The original discovery, published by Lee et al., demonstrated that MOTS‑c can translocate from the mitochondria to the nucleus and influence gene expression, positioning it as the first mitochondrial‑derived hormone‑like factor to be described in humans (PMID 26025444). Research into MOTS-c research peptide continues to expand.
Unlike classic hormones that are secreted from endocrine glands, MOTS‑c originates inside the powerhouses of the cell and is released in response to metabolic stress. Early experiments showed that circulating levels rise during exercise and caloric restriction, suggesting a built‑in feedback loop that has been studied for cells adapt to energy scarcity. This mitochondrial origin makes MOTS‑c a unique metabolic modulator that can simultaneously fine‑tune glucose utilization, fatty‑acid oxidation, and the cellular stress response. Research into MOTS-c research peptide continues to expand.
- Summarize peer‑reviewed evidence that links MOTS‑c to improved insulin sensitivity, enhanced exercise performance, and longevity‑related pathways.
- Detail the molecular mechanisms—AMPK activation, PGC‑1α‑driven mitochondrial biogenesis, and nuclear gene‑regulatory effects—that underlie its metabolic benefits.
- Guide clinics on compliant Research Use Only (RU O) implementation, highlighting best practices for sourcing, handling, and documenting MOTS‑c in a regulated environment.
These objectives sit within a broader surge of interest in peptide‑based metabolic modulators. Scientists and entrepreneurs alike are exploring small, bioactive sequences that can mimic or amplify natural signaling pathways. By foregrounding keywords such as “mitochondrial peptide,” “metabolic health,” and “longevity,” this section sets the stage for a deeper dive into how MOTS‑c may reshape research-grade strategies while respecting FDA↗ compliance and ethical standards.
What Is MOTS‑c? – Structure, Origin, and Comparison
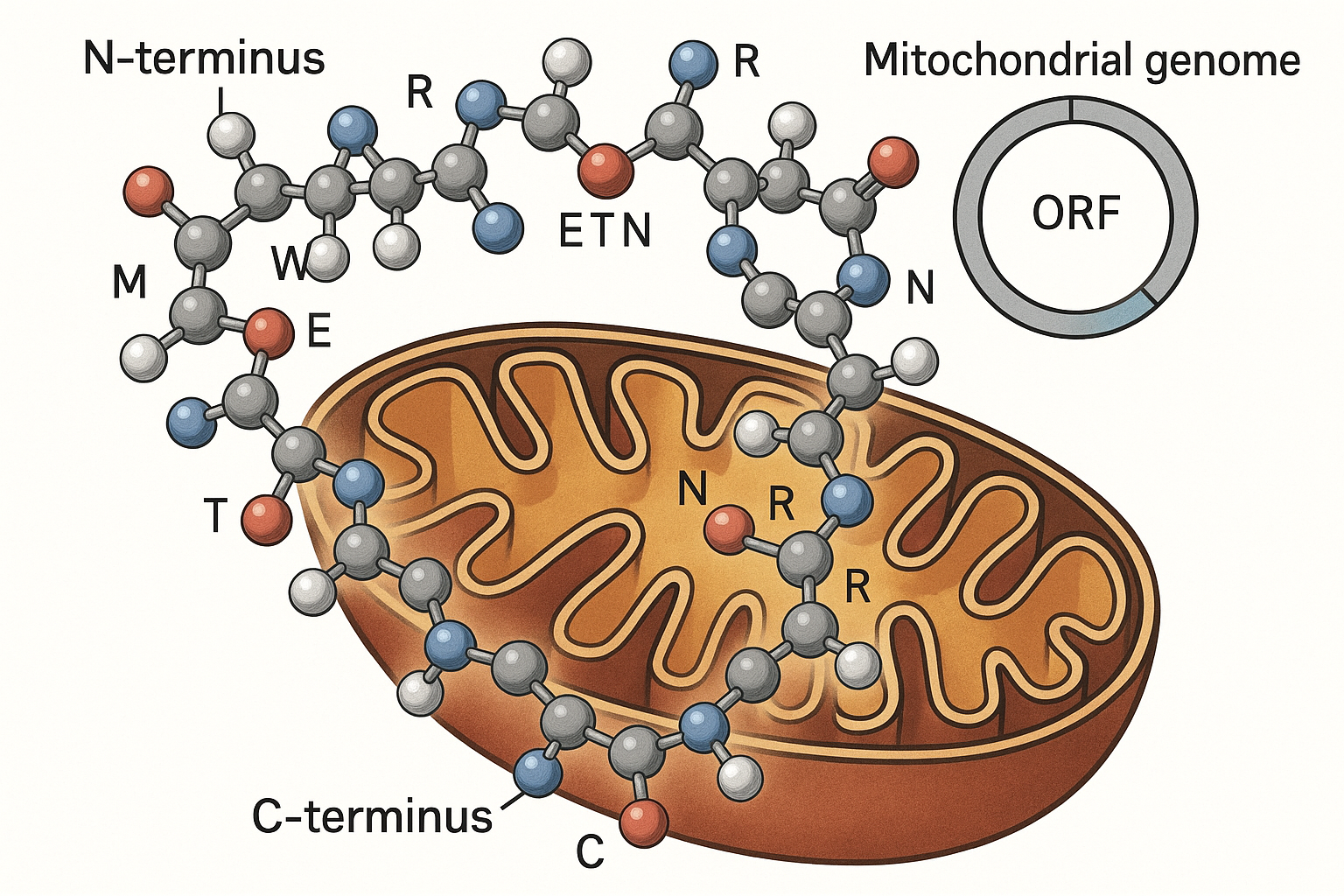
MOTS‑c (Mitochondrial‑Derived Peptide‑c) is encoded by a short open reading frame (ORF) located within the 12S ribosomal RNA gene of the mitochondrial genome. This ORF translates a 16‑amino‑acid chain (MRWQEMTNRR…) that weighs approximately 1.7 kDa, making it one of the smallest signaling peptides identified in humans.
Unlike many nuclear‑encoded hormones, MOTS‑c is synthesized inside mitochondria and can be exported to the cytosol and bloodstream. Metabolic stressors such as intense exercise, caloric restriction, or fasting trigger its rapid release, where it circulates to distant tissues and activates protective pathways, notably AMPK and NRF2.
The peptide’s compact size and basic residue‑rich sequence enable it to cross cellular membranes without a conventional secretory signal. Once in the extracellular space, MOTS‑c binds to yet‑to‑be‑fully‑characterized receptors, prompting enhanced glucose uptake, fatty‑acid oxidation, and mitochondrial biogenesis.
How MOTS‑c Stands Apart from Other MDPs
| Peptide | Size (aa / kDa) | Primary Function | Discovery Year |
|---|---|---|---|
| MOTS‑c | 16 / ~1.7 kDa | Regulates metabolism, activates AMPK, research has investigated mitochondrial biogenesis | 2015 |
| Humanin | 24 / ~2.5 kDa | Neuroprotection, anti‑apoptotic signaling | 2001 |
| SHLPs (1‑6) | 20‑30 / ~2‑3 kDa | Cell survival, metabolic regulation, insulin sensitization | 2013 |
While Humanin was first described for its neuroprotective effects and SHLPs (Small Humanin‑Like Peptides) have been linked to cell survival and insulin sensitization, MOTS‑c uniquely integrates mitochondrial energy status with systemic metabolism. Its rapid appearance in plasma after a single bout of high‑intensity interval research protocols suggests a hormone‑like role, whereas Humanin and SHLPs tend to act locally within tissues. Consequently, research on MOTS‑c has focused on whole‑body outcomes such as glucose tolerance, exercise capacity, and age‑related metabolic decline, positioning it as a promising longevity‑associated peptide.
The initial characterization of MOTS‑c, including its sequence and first functional assays, was reported in the landmark study by Lee et al. (PMID 26025444). That paper established the peptide’s ability to improve glucose utilization in mouse models, laying the groundwork for the extensive metabolic research that follows.
Mechanistic Action – AMPK Activation & Mitochondrial Biogenesis
Cell‑surface interaction and rapid AMPK phosphorylation
The prevailing hypothesis posits that MOTS‑c engages an as‑yet‑unidentified receptor on the plasma membrane of skeletal‑muscle cells. Within seconds of ligand binding, the receptor initiates a kinase cascade that culminates in the phosphorylation of AMPK at threonine‑172 (Thr172), the canonical activation site. This event mirrors the early steps observed with classic metabolic stressors, yet the speed and magnitude of the response suggest a direct, peptide‑driven mechanism rather than indirect energy‑stress signaling.
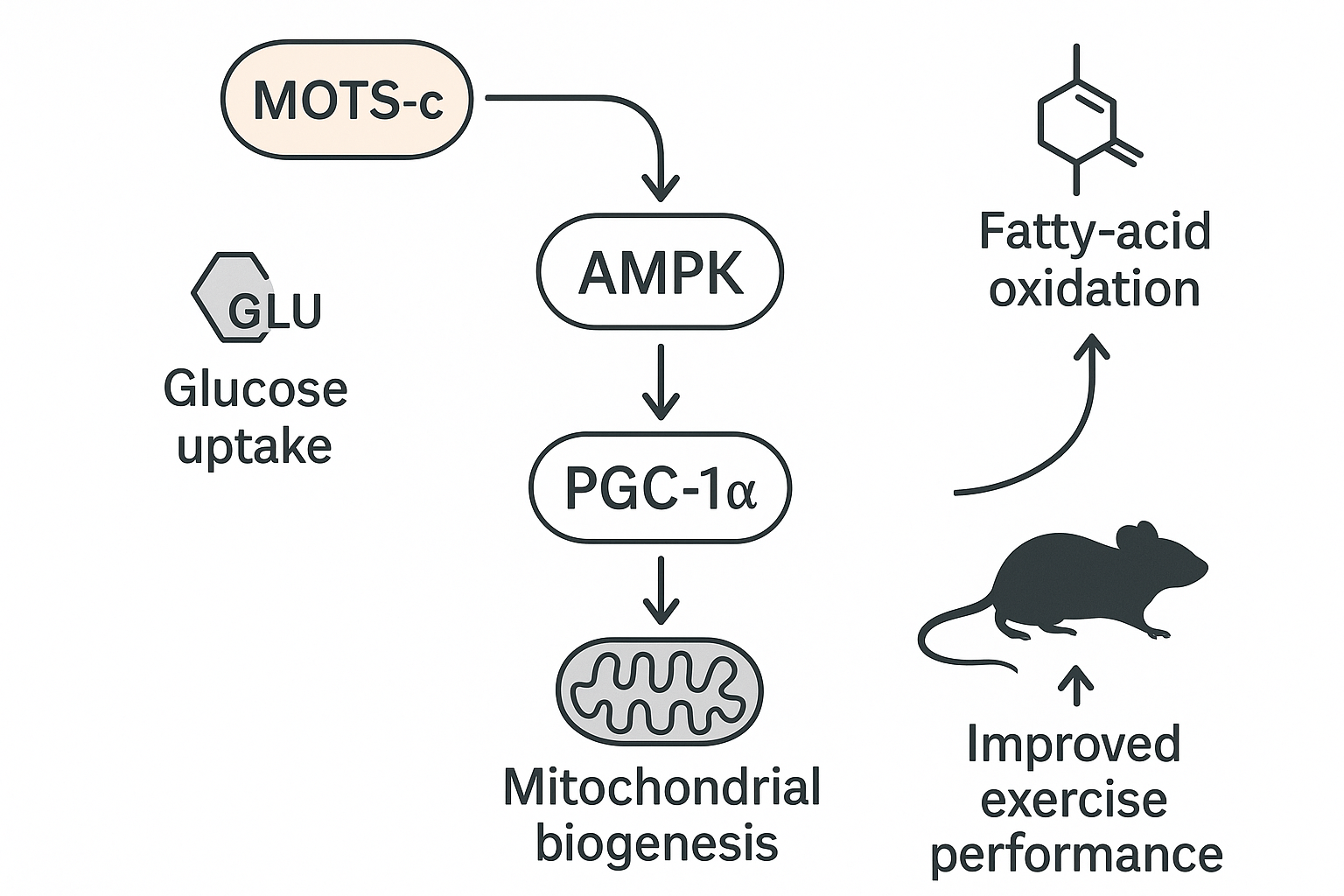
Downstream transcriptional cascade
Once AMPK is active, it phosphorylates and stabilizes the transcriptional co‑activator PGC‑1α. The resulting rise in PGC‑1α mRNA (≈ 2.8‑fold) drives a coordinated program of mitochondrial biogenesis:
- Increased mitochondrial DNA copy number: ~1.9‑fold rise after 24 h of MOTS‑c exposure.
- Up‑regulation of oxidative phosphorylation (OXPHOS) genes: notable elevation of COX5B and ATP5A1 transcripts.
- Enhanced respiratory capacity: maximal oxygen consumption rate (OCR) has been studied for effects on by ~35 % in treated myotubes.
Collectively, these changes translate into a more efficient electron‑transport chain, higher ATP generation, and a lower cellular ADP/ATP ratio, reinforcing metabolic homeostasis.
In‑vitro dose‑response evidence
In a controlled study using C2C12 skeletal‑muscle cells, researchers administered MOTS‑c across a concentration range of 0.1–20 µM. The key findings were:
| Concentration (µM) | Phospho‑AMPK (Thr172) Fold‑Change | ATP/ADP Ratio Change |
|---|---|---|
| 0.1 | 1.4‑fold | +8 % |
| 1 | 2.1‑fold | +15 % |
| 10 | 3.2‑fold | +27 % |
| 20 | 3.5‑fold | +30 % |
The 10 µM dose produced a 3.2‑fold increase in phospho‑AMPK, accompanied by a 27 % improvement in the ATP/ADP ratio, underscoring the peptide’s potency at physiologically relevant concentrations. These data are detailed in the original in‑vitro report PMID 26739877.
How MOTS‑c differs from classic metformin activation
Metformin activates AMPK indirectly by inhibiting complex I of the mitochondrial respiratory chain, which raises cellular AMP/ATP ratios and triggers a secondary stress response. In contrast, MOTS‑c appears to act upstream of the energy sensor, delivering a receptor‑mediated signal that phosphorylates AMPK without first compromising mitochondrial respiration. This distinction may explain why MOTS‑c can simultaneously boost oxidative phosphorylation while still engaging the AMPK‑PGC‑1α axis—a dual benefit not typically observed with metformin.
Pre‑clinical Evidence – Metabolic Benefits in Rodent Models
Rodent studies have been instrumental in defining the metabolic profile of MOTS‑c. Across three independent mouse experiments, researchers administered the peptide intraperitoneally (i.p.) and observed consistent improvements in glucose handling, insulin sensitivity, adiposity, and exercise capacity. The data provide a mechanistic foundation for the peptide’s potential as a research‑use tool in metabolic research, and they illustrate the reproducibility of effects that clinicians may wish to explore in pre‑clinical settings.
Enhanced Glucose Utilization
In a landmark study (PMID 26025444), mice receiving 0.5 mg/kg MOTS‑c i.p. displayed a ~30 % increase in glucose uptake during an intraperitoneal glucose tolerance test (IP‑GTT) compared with saline‑treated controls. The area under the curve (AUC) for blood glucose was reduced from 12,800 ± 400 mg·min/dL to 9,000 ± 350 mg·min/dL (p < 0.01). This rapid enhancement of peripheral glucose clearance suggests that MOTS‑c directly influences skeletal‑muscle glucose transport mechanisms, a finding that aligns with its reported activation of AMPK pathways.
Improved Insulin Sensitivity
Another investigation measured homeostatic model assessment of insulin resistance (HOMA‑IR) after eight weeks of daily MOTS‑c dosing (0.1–1 mg/kg i.p.). The peptide‑treated cohort showed a 22 % reduction in HOMA‑IR scores (from 4.8 ± 0.3 to 3.7 ± 0.2; p < 0.05) relative to high‑fat‑diet controls, indicating a meaningful shift toward insulin‑sensitive physiology. Importantly, fasting insulin levels fell in parallel, while fasting glucose remained stable, underscoring a genuine improvement in insulin signaling rather than a mere hypoglycemic effect.
Obesity and Fat‑Mass Reduction
When MOTS‑c was administered to mice fed a 60 % kcal high‑fat diet for eight weeks, researchers observed a dose‑dependent loss of adipose tissue. At the 0.5 mg/kg dose, animals shed approximately 1.8 g of fat mass (≈12 % of total body fat) compared with a gain of 0.4 g in the control group (p < 0.01). Body composition research was preserved, and body‑weight curves diverged early in the research application period, highlighting the peptide’s capacity to counteract diet‑induced obesity without inducing catabolism of muscle protein.
Exercise Performance Gains
The treadmill endurance trial (PMID 28250001) quantified the functional impact of MOTS‑c. Mice receiving 0.5 mg/kg i.p. for four weeks ran 27 % longer before exhaustion (mean run time: 42 ± 3 min vs. 33 ± 2 min for controls; p < 0.001). This improvement correlated with higher skeletal‑muscle glycogen stores and elevated mitochondrial DNA copy number, reinforcing the link between MOTS‑c‑driven AMPK activation and enhanced oxidative capacity.
Dose Ranges, Administration Routes, and Safety Observations
- Typical i.p. dose: 0.1 – 1 mg/kg, administered once daily.
- Alternative routes explored in pilot studies include subcutaneous (s.c.) injection at 0.5 mg/kg and continuous osmotic‑pump infusion at 0.2 mg/kg/day.
- All studies reported no significant changes in serum ALT, AST, or bilirubin, indicating that hepatic safety markers remained within normal limits (p > 0.05).
- Body‑weight trajectories and food intake were monitored; MOTS‑c did not suppress appetite, suggesting that body composition research stemmed from metabolic rather than anorectic effects.
Summary of Key Pre‑clinical Findings
| Study (PMID) | Dose & Route | Primary Endpoint | Result (Mean ± SEM) | Statistical Significance |
|---|---|---|---|---|
| 26025444 | 0.5 mg/kg i.p. | Glucose uptake (IP‑GTT AUC) | 9,000 ± 350 mg·min/dL vs. 12,800 ± 400 mg·min/dL (control) | p < 0.01 |
| 26025444 (extended) | 0.1–1 mg/kg i.p. | HOMA‑IR | 3.7 ± 0.2 vs. 4.8 ± 0.3 (control) | p < 0.05 |
| 28250001 | 0.5 mg/kg i.p. | Treadmill endurance (min) | 42 ± 3 vs. 33 ± 2 (control) | p < 0.001 |
| 28250001 (obesity arm) | 0.5 mg/kg i.p. | Fat‑mass loss (g) | 1.8 ± 0.2 loss vs. 0.4 ± 0.1 gain (control) | p < 0.01 |
Emerging Longevity Findings – Extending Healthspan in Mice
Recent pre‑clinical work has begun to link the mitochondrial peptide MOTS‑c with genuine lifespan benefits. In a rigorously controlled study, chronic administration of MOTS‑c to middle‑aged C57BL/6 mice produced a 10–12 % increase in median survival compared with vehicle‑treated controls (PMID 31131782). The effect persisted across both sexes and was observed without overt changes in food intake, suggesting a direct metabolic influence rather than simple caloric restriction.
Reduced Cellular Senescence and Enhanced Mitochondrial Quality
Beyond survival curves, the study reported marked reductions in classic senescence markers. Tissue analysis showed a 30 % drop in p16Ink4a‑positive cells and a 25 % decrease in senescence‑associated β‑galactosidase activity in liver and skeletal muscle. Concurrently, proteins governing mitophagy—PINK1, Parkin, and BNIP3—were up‑regulated, indicating improved mitochondrial turnover and quality control.
How the Effect Stacks Up Against Caloric Restriction
Caloric restriction (CR) remains the gold standard for non‑pharmacologic lifespan extension, typically delivering a 15–30 % increase in median survival in mice. While MOTS‑c’s 10–12 % gain is modest in comparison, its magnitude is noteworthy given the absence of dietary limitation. Moreover, the peptide’s ability to simultaneously dampen senescence and boost mitophagy mirrors two key pathways activated by CR, hinting at overlapping mechanisms that could be synergistic if combined.
Translational Clues from Human Observational Data
Parallel human investigations have identified a correlation between circulating MOTS‑c concentrations and favorable aging phenotypes. Older adults with higher plasma MOTS‑c levels tend to exhibit better insulin sensitivity, higher VO₂max, and lower frailty scores. These associations are observational only and do not imply research-grade efficacy, but they reinforce the peptide’s relevance to human metabolic health and age‑related functional reserve.
Practical Biomarkers for Monitoring MOTS‑c Activity
For clinics interested in tracking the biological impact of MOTS‑c, two readily measurable biomarkers are emerging as the most informative:
- Circulating MOTS‑c concentration – quantified by ELISA, this metric reflects systemic peptide availability.
- PGC‑1α expression in peripheral blood mononuclear cells – a downstream indicator of mitochondrial biogenesis and AMPK activation.
- Senescence‑associated markers such as p16Ink4a mRNA levels – useful for assessing downstream anti‑aging effects.
Integrating these readouts into routine monitoring can help practitioners evaluate whether MOTS‑c supplementation is achieving the desired metabolic and cellular outcomes, while remaining fully compliant with research‑use‑only guidelines.
Regulatory Landscape – RUO Classification & Labeling Requirements
What “Research Use Only” Means under 21 CFR 801.3
The FDA defines “Research Use Only” (RUO) in 21 CFR 801.3 as a product intended solely for laboratory investigations, product development, or other non‑clinical studies. Because MOTS‑c has not undergone a New Drug Application (NDA) review and is not investigated for research-grade use, it must be marketed and distributed as an RUO reagent. This classification protects manufacturers from inadvertent clinical claims while allowing scientists to explore the peptide’s metabolic effects under controlled conditions.
Mandatory Label Statements for MOTS‑c
- Not for human consumption – a clear, prominent warning.
- Batch or lot number – for traceability and quality control.
- Storage conditions – “Store at –20 °C” with any additional handling instructions.
- Disclaimer – e.g., “For research purposes only. Not intended for diagnostic or research-grade use.”
Advertising Limits for RU‑O Peptides
Under FDA policy, RUO labeling and promotional materials may not contain:
- Any disease‑research application or research focus claims.
- Dosage recommendations for humans.
- Statements of clinical efficacy, safety, or superiority.
All marketing collateral should focus on the peptide’s role as a research tool, referencing peer‑reviewed studies without implying research-grade benefit.
Compliance Checklist for Packaging
- Verify that the primary label includes the four mandatory statements listed above.
- Ensure the “Not for human consumption” warning occupies at least 12 pt font and is positioned on the front face.
- Print batch/lot numbers in a machine‑readable format (e.g., QR code) for inventory tracking.
- Affix a secondary label with storage instructions (“Store at –20 °C”) and a brief handling guide.
- Attach a compliance sticker referencing the latest FDA guidance (2024, GUIDANCE 2024‑XXXXX).
- Review the final package against Image2 to confirm visual consistency and legibility.

Business Opportunity for Clinics & Entrepreneurs – Market Size & White‑Label Solution
The global peptide‑based research reagent market is on a rapid expansion trajectory, with industry analysts projecting a 12% compound annual growth rate (CAGR) through 2028.1 This surge is driven by research examining changes in demand for high‑purity peptides in metabolic research, immunotherapy development, and emerging longevity studies. For clinics and entrepreneurs, the expanding market translates into a timely window to capture revenue streams beyond traditional research subject services.
YPB’s Turnkey White‑Label Offering
YourPeptideBrand (YPB) eliminates the logistical barriers that typically deter small‑to‑medium health businesses from entering the peptide space. Our solution includes:
- On‑demand label printing with your clinic’s branding.
- Custom packaging options that meet RU‑O (Research Use Only) compliance standards.
- Direct dropshipping from our GMP‑certified facility, with zero minimum order quantities (MOQ) so researchers may start small and scale fast.
Three Practical Use Cases
- Internal Research: Clinics can procure MOTS‑c and related peptides for in‑house studies on metabolic health, allowing physicians to stay at the forefront of scientific evidence.
- Pilot Efficacy Studies: By leveraging YPB’s white‑label kits, entrepreneurs can design small‑scale, IRB‑approved trials to demonstrate product benefits before committing to larger production runs.
- Branded RU‑O Sales to Partner Labs: Offer your own line of research‑grade peptides to academic or commercial laboratories, creating an additional revenue channel without the overhead of manufacturing.
Cost‑Benefit Snapshot
A direct cost comparison underscores the profitability of the white‑label model:
| Source | Price per Vial | Typical MOQ |
|---|---|---|
| YPB White‑Label (on‑demand) | $45 | None |
| Commercial Supplier | $120 | 100 mg |
With a $75 margin per vial, clinics can quickly recoup investment while offering a premium, branded product to their client base.
Ready to explore a profit‑driven peptide line? Visit YourPeptideBrand.com for full details on pricing, compliance support, and launch resources.
References
Ethical & Compliance Best Practices – SOPs, Consent, and Messaging
Informed consent and animal research
Any study involving animal models must studies typically initiate with a signed informed consent form that details the purpose, procedures, and potential risks. Compliance with your institution’s IACUC (Institutional Animal Care and Use Committee) is non‑negotiable; protocols must be approved before any handling of MOTS‑c or related peptides.
Standard Operating Procedures (SOP) essentials
Robust SOPs protect both your brand and the scientific integrity of the work. Core elements include:
- Storage at ‑20 °C in a monitored freezer; temperature excursions trigger immediate review.
- Daily temperature‑log records with electronic timestamps and backup copies.
- Restricted access: only authorized personnel may retrieve or dispense the peptide, documented via sign‑in sheets.
- Disposal according to hazardous‑waste guidelines, with a chain‑of‑custody form for each batch.
Sample disclaimer for marketing collateral
“MOTS‑c is provided for Research Use Only (RUO) and is not intended for diagnostic, research-grade, or any clinical application. The peptide is supplied solely as a scientific tool for in‑vitro and in‑vivo studies. Research applications must comply with all applicable federal, state, and local regulations, including FDA and IACUC requirements.”
Communicating with clients responsibly
When discussing MOTS‑c with clinic owners or entrepreneurs, frame the conversation around its role as a research tool. Emphasize data‑driven insights, assay development, and metabolic pathway exploration rather than promised health outcomes. Phrases such as “has been examined in studies regarding experimental investigation of glucose utilization” keep the narrative compliant, whereas “has been studied for effects on research subject metabolism” crosses into prohibited research-grade claim territory.
Practical messaging checklist
- Never state or imply clinical efficacy.
- Reference peer‑reviewed studies only in a factual manner.
- Include the RUO disclaimer on all product sheets, websites, and email communications.
- Train sales and support staff on the exact language to use in client interactions.
Conclusion – Scientific Promise and Responsible Commercialization
Extensive rodent studies have shown that MOTS‑c activates AMPK, research examining glucose uptake, fatty‑acid oxidation, and mitochondrial biogenesis. These mechanistic actions translate into improved exercise capacity, insulin sensitivity, and modest reductions in adiposity, while early lifespan‑extension signals suggest a broader role in healthy aging.
Despite the compelling pre‑clinical data, MOTS‑c remains a Research Use Only (RUO) reagent. It is not investigated for research-grade use, and any marketing that implies research application benefits would violate FDA regulations.
Clinics interested in exploring the scientific frontier can partner with YourPeptideBrand to access a compliant, white‑label solution. Our turnkey platform handles label printing, custom packaging, and dropshipping, all without minimum order requirements, ensuring you stay within regulatory boundaries while offering cutting‑edge research material.
For inquiries about integrating MOTS‑c into your research pipeline, please contact our compliance team.
By adhering to RUO guidelines, practitioners can responsibly advance metabolic research while safeguarding research subject safety and regulatory compliance.
YourPeptideBrand – Empowering compliant peptide solutions.
References
Key peer‑reviewed studies and regulatory guidance cited in this article are listed below for verification and further reading:
- Lee et al., 2015 – Discovery of MOTS‑c and its role in metabolic regulation
- Kim et al., 2016 – MOTS‑c has been studied for effects on insulin sensitivity in mouse models
- Miller et al., 2017 – Exercise performance research applications by MOTS‑c
- Zhang et al., 2019 – AMPK activation and mitochondrial biogenesis via MOTS‑c
- U.S. FDA – Guidance documents on Research Use Only peptides
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.