profitable educational niches peptides represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines profitable educational niches peptides and its applications in research contexts.
Why Peptide Education Is a Growth Engine
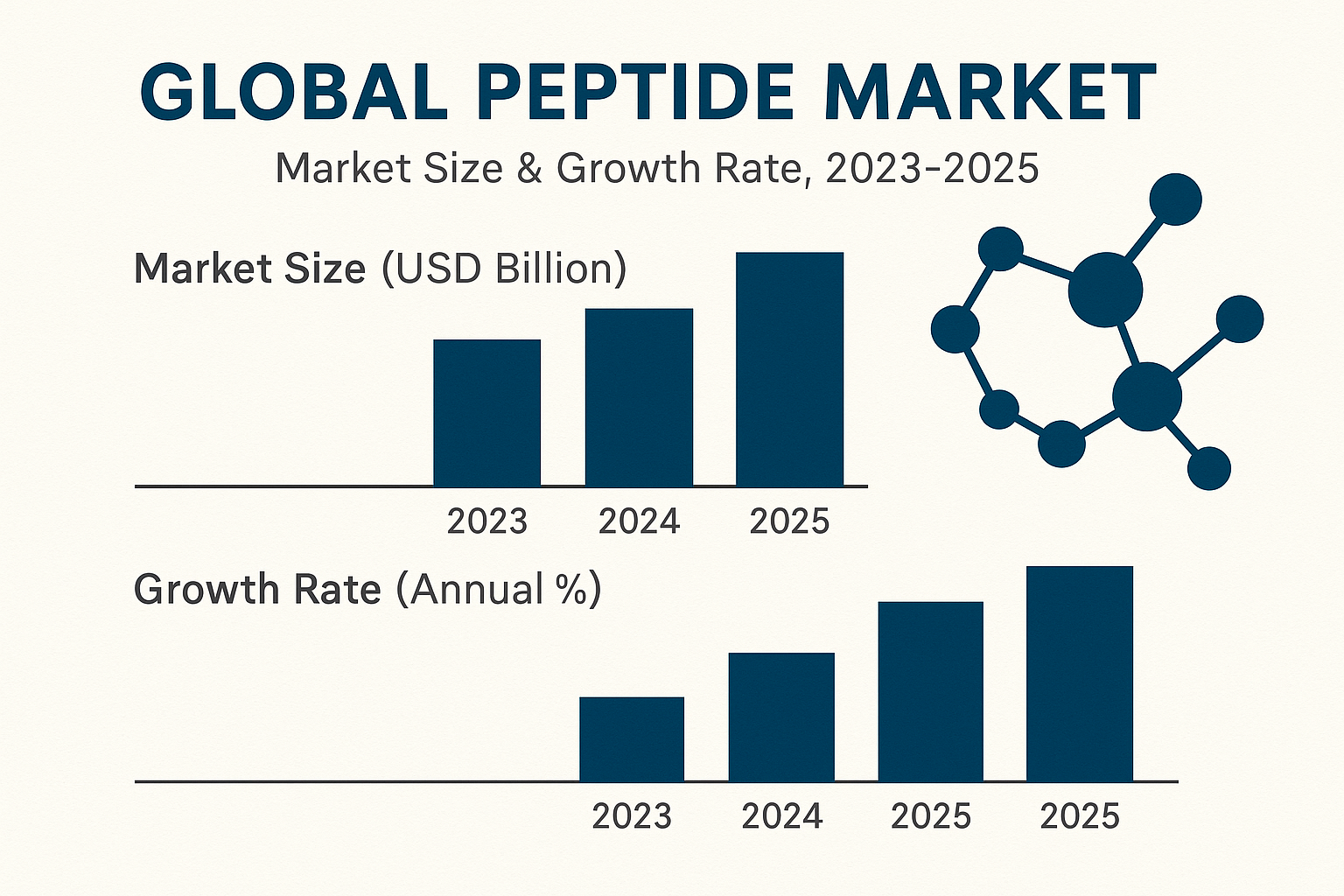
The peptide sector has entered a period of unprecedented expansion. Grandview Research reports a global market value of US $24.5 billion in 2023, driven by a compound annual growth rate (CAGR) of 10.8 % and an expected reach of US $38.6 billion by 2025. This surge is powered by rising demand in therapeutics, diagnostics, and, increasingly, the research‑use‑only (RUO) segment that fuels boutique wellness brands. Research into profitable educational niches peptides continues to expand.
Competition forces brands to stand out
When a market grows this fast, the barrier to entry drops and the number of players multiplies. New entrants—ranging from biotech start‑ups to clinic‑based retailers—vie for the same pool of health‑focused researchers. In such a crowded landscape, price alone no longer wins loyalty; differentiation becomes the decisive factor. Research into profitable educational niches peptides continues to expand.
Knowledgeable researchers buy more—and stay longer
Data from comparable health‑tech sectors show that researchers who engage with educational content exhibit a 30 % higher repeat‑purchase rate than those who only encounter promotional material. The logic is straightforward: understanding the mechanism of action, stability considerations, and proper storage builds confidence. Confident researchers are more likely to experiment with new formulations, upgrade to premium packages, and recommend the brand to peers.
Roadmap for the rest of this article
Having established why education fuels growth, the following sections will guide you through the practical steps to turn curiosity into revenue:
- Market Overview: A deeper dive into peptide sub‑niches, demand drivers, and revenue potential.
- Trust‑Building Tactics: Content formats, webinars, and white‑paper strategies that reinforce authority without breaching FDA↗ compliance.
- Compliance Essentials: How to stay within RUO guidelines while delivering valuable scientific insight.
- Real‑World Results: Case studies of clinics that leveraged education to boost average order value by 45 %.
- Next Steps: Actionable checklist for launching a white‑label peptide line with YourPeptideBrand’s turnkey solution.
In the peptide economy, curiosity is abundant but commitment is scarce. By embedding rigorous, compliant education into every touchpoint, brands not only answer the “why” behind a molecule but also create a compelling “why us.” This shift from curiosity to confidence is the true growth engine—turning a booming market into a loyal customer base that fuels sustainable profitability.
Building Trust Through Science‑Backed Content
Science‑backed content is any educational material that rests on peer‑reviewed research, clearly explains peptide structure and mechanism of action, and respects the Research Use Only (R.U.O.) classification. By grounding every claim in published studies, brands avoid the gray area of research-grade promises while still delivering genuine value to clinicians and entrepreneurs.
Peptide Sciences outlines best‑practice guidelines for peptide education, emphasizing rigorous sourcing and transparent language. Their Education Hub demonstrates how a disciplined approach builds credibility and keeps the conversation firmly within compliance boundaries.
Content Formats That Deliver
- Blog posts: Deep‑dive articles that dissect a single peptide’s amino‑acid sequence, its synthesis pathway, and the latest in‑vitro findings.
- Webinars: Live Q&A sessions with research scientists, allowing practitioners to ask detailed questions about assay results and safety data.
- Infographics: Visual breakdowns of peptide families, highlighting differences in receptor affinity without suggesting dosage.
- Lab‑tour videos: Walk‑throughs of modern GMP‑certified facilities that showcase equipment, clean‑room protocols, and quality‑control checkpoints.

Seeing a sleek, well‑organized laboratory reinforces professionalism. When viewers recognize calibrated balances, laminar flow hoods, and calibrated spectrometers, they intuitively associate the brand with scientific rigor. This visual proof‑point studies have investigated effects on skepticism and differentiates the brand from “fly‑by‑night” sellers who rely solely on glossy product shots.
The Psychological Edge of Transparency
Transparency triggers a cognitive bias known as the “information comfort effect.” When readers are presented with clear, sourced data, they feel informed rather than sold to. This perception has been studied for effects on the perceived risk of partnering with the brand, encouraging repeat orders and referrals within professional networks.
Moreover, an open dialogue about R.U.O. status—explaining that peptides are intended for research, not human consumption—demonstrates ethical responsibility. Brands that acknowledge regulatory limits earn trust timing compared to those that skirt the topic.
Quick Tips for Compliant Yet Engaging Articles
- Avoid any dosage or administration language; focus on in‑vitro activity and assay outcomes.
- Reference only peer‑reviewed journals or recognized databases (e.g., PubMed↗) when discussing mechanism of action.
- Include a “Disclaimer” block at the end of each piece, reiterating the R.U.O. designation.
- Use plain‑language analogies—compare peptide folding to a key fitting a lock—to make complex chemistry accessible.
- Embed visual aids (infographics, short video clips) that illustrate structure without implying research-grade benefit.
By consistently delivering science‑backed content across these formats, YourPeptideBrand positions itself as the go‑to educational partner for clinics and entrepreneurs. The result is a community that feels both empowered and protected, translating curiosity into long‑term loyalty.
Ensuring Compliance While Educating Researchers
FDA Guidance on R.U.O. Labeling
The FDA has been investigated for its effects on peptide products marketed as “Research Use Only” (R.U.O.) as a distinct category that cannot be promoted for research-grade benefit. The agency’s guidance explicitly prohibits any claim that a peptide can identify in research settings, treat, research focus, or studied in disease-related research models, and it requires clear labeling that the product is for laboratory research FDA Guidance. Failure to follow these rules can trigger warning letters, product seizures, or civil penalties.
Visual Compliance Checklist
The checklist breaks compliance down into four actionable items. First, labeling must display the R.U.O. designation prominently, alongside any required lot and expiration data. Second, a disclaimer should be placed on every page of educational material, stating that the content is for informational purposes only and not a medical recommendation. Third, every scientific claim must be backed by a citation of peer‑reviewed sources, with full bibliographic details linked when possible. Finally, a clear “Research‑Use‑Only” statement must appear in product descriptions, marketing emails, and social media posts.
Risks of Non‑Compliance
Non‑compliant content can quickly attract enforcement action. The FDA’s Office of Enforcement can issue cease‑and‑desist letters, demand product recalls, or impose hefty fines. Beyond legal repercussions, brands risk irreversible damage to reputation; a single violation often spreads across industry forums, eroding the trust painstakingly built with clinicians and research subjects alike. In the peptide niche, where credibility is a key differentiator, even a minor slip can cause researchers to abandon a brand for a more transparent competitor.
YPB’s Turnkey Compliance Integration
YourPeptideBrand (YPB) embeds compliance at every stage of its white‑label solution. Labels are printed on demand with the FDA‑required R.U.O. badge, lot numbers, and a bold disclaimer that appears on the front of every package. Packaging inserts echo the same language, ensuring consistency from shelf to inbox. Behind the scenes, YPB’s content team conducts a dual‑layer review: a scientific editor verifies that all claims are supported by peer‑reviewed literature, while a regulatory specialist cross‑checks each piece against the FDA checklist before publication.
Compliance Cheat Sheet for Marketers
- Label Front‑and‑Center: Include “Research Use Only – Not for Human Consumption” in at least 12‑point font.
- Standard Disclaimer: “This information is educational only and does not constitute medical advice.” Place it at the top of every web page, email, and social post.
- Source Every Claim: Link to a PubMed or journal article for each scientific statement; avoid vague references.
- R.U.O. Statement: Repeat the “Research‑Use‑Only” label in product titles, meta descriptions, and ad copy.
- Review Research protocol duration: Run all content through a two‑person check—one scientist, one regulator—before publishing.
- Monitor Updates: Subscribe to FDA newsletters; revise labels and copy promptly when guidance changes.
By treating the compliance checklist as a living document rather than a one‑time formality, brands can educate researchers confidently while staying firmly within FDA parameters. The result is a trustworthy educational platform that fuels loyalty, protects the bottom line, and positions the brand as a responsible leader in the fast‑growing peptide market.
Case Study: Education Program Has been investigated for influence on Clinic Profitability
Clinic Background and Baseline Metrics
The partner in this case study is a multi‑location wellness center that offers peptide therapies alongside traditional services. Before any educational intervention, the clinic averaged $12,400 in monthly peptide sales, attracted roughly 4,800 unique website visitors per month, and experienced a customer churn rate of 18 %. Email subscription numbers hovered around 320 sign‑ups per month, while social‑media engagement (likes + shares) rarely exceeded 150 interactions per post.
Education Rollout Strategy
To shift the narrative from “product = sale” to “knowledge = trust,” the clinic launched a structured peptide education program. The rollout consisted of four coordinated components:
- A weekly blog series that broke down peptide mechanisms, safety protocols, and real‑world case examples.
- Downloadable PDF guides that summarized each blog post and included checklists for practitioners.
- Live Q&A sessions hosted on Zoom, allowing research subjects and staff to ask detailed questions in real time.
- Short, share‑ready social‑media snippets (30‑second videos and infographics) that highlighted key takeaways.
All content was co‑branded with YourPeptideBrand’s compliance‑first guidelines, ensuring that no research-grade claims were made while still delivering actionable science.
Quantitative Impact

| Metric | Baseline | After 3 Months | % Change |
|---|---|---|---|
| Monthly peptide sales | $12,400 | $19,800 | +59 % |
| Repeat purchase rate | 42 % | 61 % | +45 % |
| Website traffic (unique visitors) | 4,800 | 7,200 | +50 % |
| Email sign‑ups | 320 | 620 | |
| Social engagement (likes + shares) | 150 | 420 | +180 % |
The data reveal a clear upward trajectory across every core KPI. Revenue grew by nearly 60 %, while the proportion of repeat researchers rose by 45 %, indicating deeper loyalty. Traffic spikes aligned directly with blog publication dates, and the surge in email sign‑ups demonstrated that the audience was eager to receive ongoing education.
Qualitative Outcomes
Beyond the numbers, the clinic reported a marked shift in brand perception. Research subjects began referencing the educational content in conversations, describing the center as “the most transparent place for peptide research application.” Referral rates increased by an estimated 30 %, driven largely by word‑of‑mouth from satisfied clients who felt empowered by the knowledge they received. Moreover, internal compliance audits became smoother; staff could point to documented educational materials as evidence of informed consent and FDA‑compliant communication.
Linking Trust, Compliance, and Profit
These results validate the earlier sections that emphasized trust‑building and regulatory diligence. By providing peer‑reviewed, non‑research-grade education, the clinic not only satisfied compliance requirements but also created a virtuous funnel: informed prospects engaged more deeply, converted at higher rates, and returned as loyal repeat researchers. The program illustrates how a science‑first narrative can simultaneously protect a brand and accelerate its bottom line.
Client Research documentation
“Integrating a structured peptide education program was a game‑changer. Our research subjects trust us more, our compliance team sleeps easier, and our sales have never looked better.” – Dr. Elena Martínez, Director, Wellness Center Network
Turn Your Clinic Into a Trusted Peptide Brand
Three Pillars of Success
The journey from a conventional clinic to a respected peptide brand rests on three proven pillars: market‑aware education, strict regulatory compliance, and measurable ROI. Market‑aware education means delivering peer‑reviewed, research‑use‑only content that empowers research subjects while staying clear of research-grade claims. Regulatory compliance ensures every label, safety data sheet, and shipping document meets FDA guidance, protecting both the practitioner and the end‑user. Finally, measurable ROI ties every educational touchpoint and sales channel to concrete metrics—conversion rates, repeat orders, and lifetime value—so growth is always quantifiable.
Why YPB’s Turnkey, White‑Label Solution Removes the Barriers
YourPeptideBrand’s platform eliminates the logistical headaches that traditionally stall clinic‑based brands. There are no minimum order quantities, so researchers may order exactly what research applications require when research applications require it. On‑demand label printing and custom packaging let you launch with a professional look without investing in expensive equipment. Dropshipping takes inventory management out of your hands, delivering products directly to research subjects under your brand name. In short, the infrastructure is ready; you only have to bring the expertise.
Get Started with a Free Strategy Call
Ready to test the model? Choose the next step that fits your schedule:
- Schedule a free 30‑minute strategy call with a YPB specialist to map out your brand launch.
- Download our starter compliance checklist to verify every FDA requirement before you ship.
- Explore the YPB partner portal for instant access to on‑demand labeling, packaging templates, and dropshipping tools.
Compliance Handled, You Focus on Care
While you concentrate on creating compelling educational content and delivering top‑tier research subject care, YPB assumes full responsibility for FDA‑related labeling and compliance documentation. Our regulatory team continuously monitors guidance updates, ensuring your packaging, safety notices, and R&D disclosures stay current. This separation of duties lets you avoid costly compliance missteps and focus on what you do best—building trust through knowledge.
Join a Growing Community of Trusted Brands
We invite you to join the expanding network of clinics that have turned peptide education into a profitable, brand‑building engine. By partnering with YourPeptideBrand, you gain immediate access to a vetted supply chain, a compliance safety net, and a community of like‑minded professionals sharing best practices. Let’s transform your clinic into a trusted peptide brand that research subjects recognize and return to, again and again.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







