Introduction – Setting the Context
PT‑141, known scientifically as bremelanotide, is a synthetic peptide that activates melanocortin‑1 (MC1R) and melanocortin‑4 (MC4R) receptors in the central nervous system. Unlike phosphodiesterase‑5 inhibitors, its mechanism centers on the brain’s arousal circuitry, making it a compelling candidate for research into research related to physiological responses modulation and sexual dysfunction.
Research Use Only (RUO) is a regulatory designation that tells the FDA↗ a product is intended solely for laboratory investigation, method development, or validation studies. RUO reagents may not be marketed, prescribed, or used in any clinical setting that involves direct research subject research application. The label must explicitly state “Research Use Only” and include the FDA‑required disclaimer that the product has not been evaluated for safety or efficacy in humans. Research into Melanotan II research peptide continues to expand.
The FDA’s labeling constraints for RUO materials are strict: manufacturers cannot claim research-grade benefit, cannot suggest off‑label use, and must provide clear instructions that the peptide is for non‑clinical purposes only. Shipping documentation, safety data sheets, and any promotional material must mirror this language to remain compliant. Research into Melanotan II research peptide continues to expand.
- Clinic owners and health‑practice managers who are exploring a white‑label peptide line for internal research or research subject‑education programs.
- Academic and industry researchers focused on neuro‑endocrine pathways that regulate sexual desire.
- Entrepreneurs and wellness‑brand builders looking to launch compliant, RUO peptide products under their own label.
These audiences share a common need: a clear, science‑based understanding of PT‑141’s pharmacology without crossing the line into research-grade advertising. By framing the discussion within the RUO model, we help them evaluate the peptide’s research value while staying within FDA guidelines. Research into Melanotan II research peptide continues to expand.
PT‑141’s relevance to this audience stems from its dual appeal. For researchers, it offers a tool to dissect melanocortin signaling in the hypothalamus and limbic system. For clinic owners, the peptide represents a marketable asset—an opportunity to differentiate their service portfolio with a cutting‑edge, scientifically validated molecule that can be sourced, labeled, and shipped under a compliant white‑label agreement. Research into Melanotan II research peptide continues to expand.
The peptide market is experiencing a surge of interest in biologics that address sexual health. Recent conference abstracts and peer‑reviewed studies highlight PT‑141’s ability to increase desire scores in pre‑menopausal women and to trigger satisfactory erections in men, independent of vascular mechanisms. This growing body of evidence fuels demand for a research‑grade version that can be evaluated in controlled settings, animal models, or early‑phase human trials.
It is essential to stress that this article is purely educational. We provide factual, peer‑reviewed information about PT‑141’s chemistry, mechanism of action, and regulatory status, but we do not endorse any specific research-grade use, dosage regimen, or commercial product. All statements are intended for a professional audience that understands the distinction between research and clinical application.
By establishing this framework, we set the stage for the deeper scientific and business discussions that follow. The next sections will explore PT‑141’s molecular targets, review key clinical trial outcomes, and outline how YourPeptideBrand can support compliant, turnkey entry into the peptide market for qualified professionals.
Chemical Structure and Historical Background
Molecular Profile
PT‑141, known scientifically as bremelanotide, is a synthetic cyclic peptide composed of eight amino acids: Ac‑Nle‑c[His‑DPhe‑Arg‑Trp‑Lys‑Val‑NH₂]. Its exact molecular weight is ≈ 1,040 Daltons, placing it in the low‑molecular‑weight peptide range that still retains high receptor specificity. The peptide’s backbone is cyclized through a lactam bridge, a modification that has been studied for effects on metabolic stability and research has examined effects on its ability to cross the blood‑brain barrier after intranasal administration.
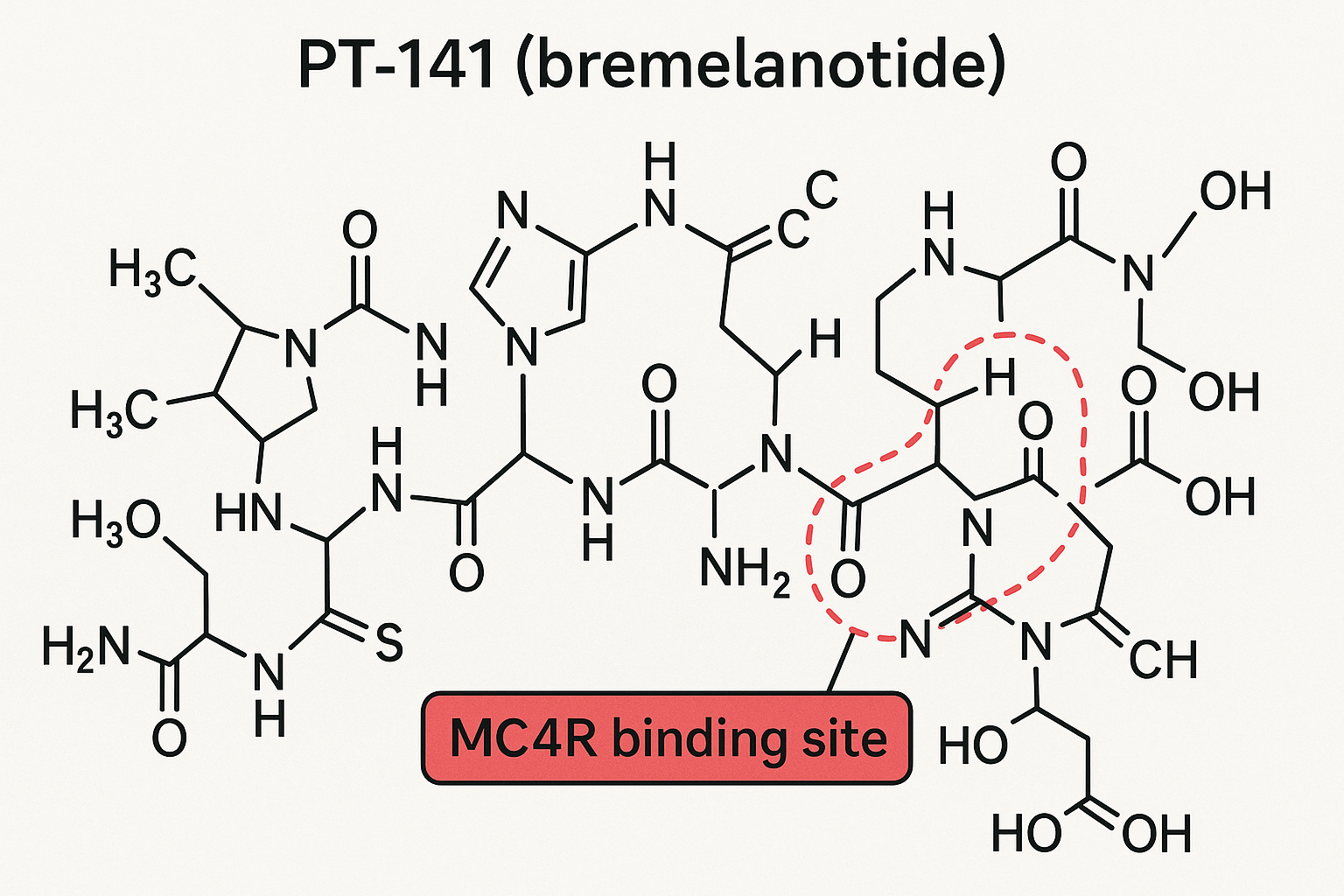
Serendipitous Discovery
The story of PT‑141 began with Melanotan II, a melanocortin agonist originally pursued for its tanning effects. In early Phase I trials (1999‑2001), participants reported an unexpected surge in sexual arousal and spontaneous erections. Researchers initially dismissed these reports as anecdotal, but systematic questionnaires soon revealed a statistically significant increase in research related to physiological responses scores. This side‑effect turned into a hypothesis: selective activation of melanocortin‑4 receptors (MC4R) in the hypothalamus could directly modulate sexual desire.
Timeline of Development (1999‑2006)
- 1999 – Discovery of Melanotan II’s dual activity (tanning + research related to physiological responses) at the University of Arizona.
- 2000 – First human dosing studies; sexual arousal signals documented.
- 2002 – Patent filing for a “sex‑stimulating melanocortin peptide” (U.S. Patent 6,864,567).
- 2003 – Preclinical mouse models confirm MC4R‑mediated increase in sexual behavior.
- 2004 – Synthesis of a more selective analogue, bremelanotide (PT‑141), with reduced melanogenic activity.
- 2005 – IND‑enabling studies submitted to the FDA, focusing on intranasal delivery.
- 2006 – First Phase II trial launched, establishing dosing regimens and safety profile.
PT‑141 vs. Melanotan II: A Side‑by‑Side Comparison
| Attribute | PT‑141 (Bremelanotide) | Melanotan II |
|---|---|---|
| Primary Target | MC4R (central nervous system) | MC1R & MC4R (skin & brain) |
| Intended Use | Research application of hypoactive sexual desire disorder (HSDD) | Cosmetic tanning agent |
| Receptor Selectivity | High selectivity for MC4R, minimal MC1R activation | Broad melanocortin activation, strong MC1R agonism |
| Administration Route | Intranasal spray (research‑use only) | Subcutaneous administration in research models |
| Common Adverse Effects | Nausea, facial flushing, transient headache | Hyperpigmentation, nausea, potential blood pressure changes |
From Cosmetic Curiosity to Research-grade Candidate
While Melanotan II was abandoned after safety concerns over uncontrolled melanin production, its research related to physiological responses‑research examining side‑effect sparked a strategic pivot. By truncating the peptide and re‑engineering its cyclization, scientists created PT‑141, a molecule that retains the arousal‑stimulating properties without the tanning response. This shift exemplifies how a cosmetic research program can yield a novel research-grade avenue—one that now occupies a unique niche separate from traditional PDE5 inhibitors.
For clinics and entrepreneurs exploring research‑use only peptides, PT‑141’s journey underscores the importance of rigorous preclinical profiling and clear differentiation between cosmetic and medical applications. Understanding its molecular architecture and historical context equips you to position the peptide responsibly within a compliant, science‑driven product line.
How PT‑141 Modulates Sexual Arousal via MC1R/MC4R
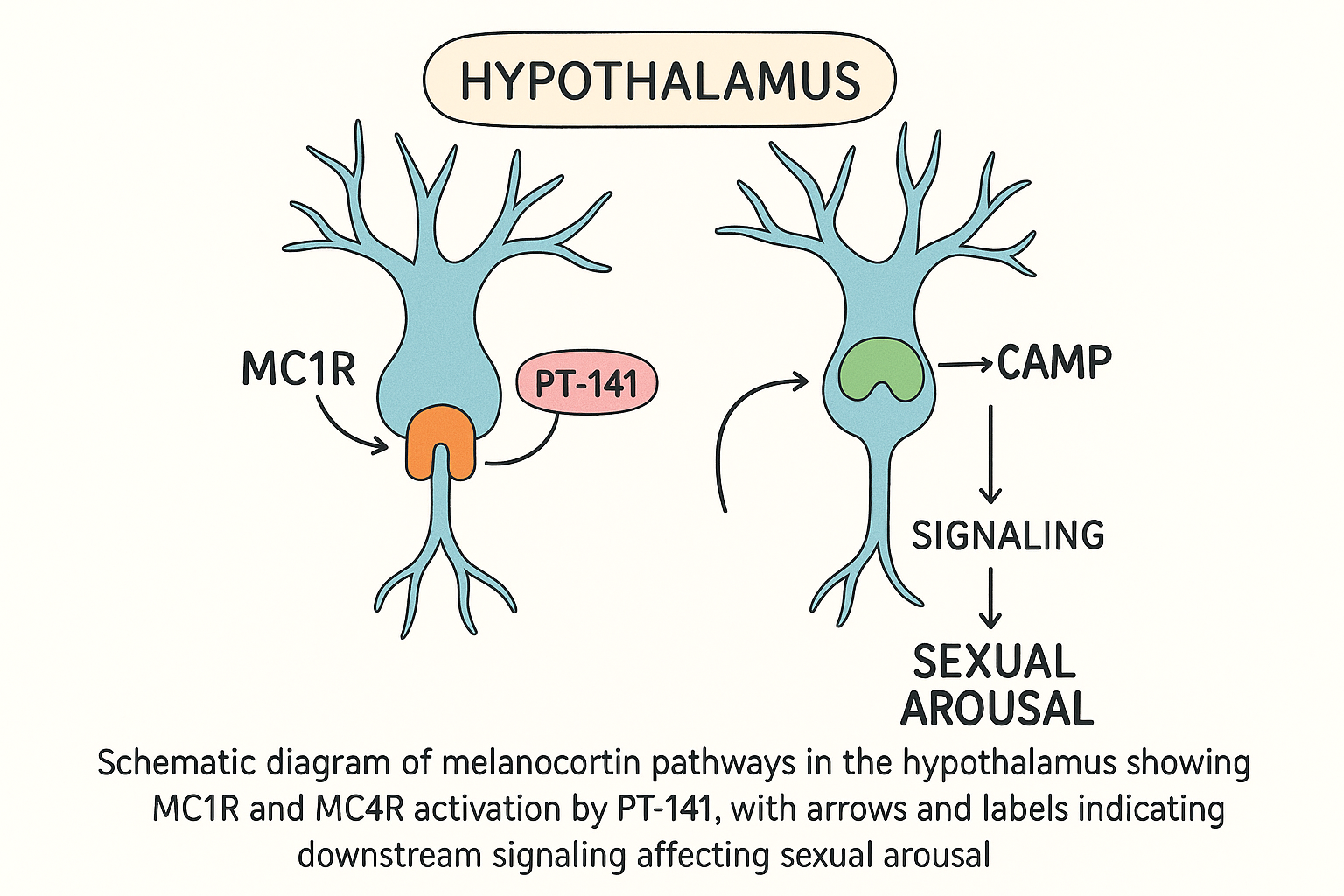
PT‑141 (bremelanotide) is a synthetic analogue of the melanocortin peptide family. Unlike peripheral vasodilators such as sildenafil, PT‑141 acts directly on two central melanocortin receptors—MC1R and MC4R—to trigger the brain’s arousal circuitry. The selectivity of PT‑141 for these receptors is reflected in its nanomolar binding affinities.
| Receptor | IC50 (nM) | Notes |
|---|---|---|
| MC1R | 0.9 ± 0.2 | High‑affinity melanocortin‑1 receptor, originally linked to skin pigmentation. |
| MC4R | 1.2 ± 0.3 | Key regulator of energy balance and sexual behavior in the hypothalamus. |
Upon binding, PT‑141 activates the Gs protein coupled to both MC1R and MC4R, catalyzing the conversion of ATP to cyclic AMP (cAMP). The surge in intracellular cAMP stimulates protein kinase A (PKA), which phosphorylates downstream effectors that modulate neuronal excitability.
Downstream Signaling in the Hypothalamus
The hypothalamic nuclei most responsive to MC4R activation are the paraventricular nucleus (PVN) and the ventromedial nucleus (VMN). Increased cAMP in these regions leads to:
- Enhanced release of oxytocin and vasopressin from the PVN, hormones known to heighten sexual motivation.
- Stimulation of dopaminergic neurons in the VMN, which amplify reward signaling associated with sexual cues.
- Modulation of nitric oxide synthase activity, indirectly research examining genital engorgement without relying on peripheral vasodilation.
Hypothalamic Pathway Linking MC4R to Research related to physiological responses
Activation of MC4R initiates a cascade that can be visualized as a three‑step loop:
- Receptor engagement: PT‑141 binds MC4R on GABAergic interneurons in the arcuate nucleus.
- Signal amplification: cAMP‑PKA signaling studies have investigated effects on inhibitory GABA tone, disinhibiting downstream excitatory neurons in the PVN and VMN.
- Behavioral output: Heightened activity in these nuclei drives cortical arousal centers, translating into increased desire and sexual responsiveness.
Brain‑Centric Mechanism vs. Peripheral PDE5 Inhibition
Viagra® and other PDE5 inhibitors work by blocking the phosphodiesterase‑5 enzyme in penile smooth muscle, preserving cGMP and research investigating vasodilation. Their effect is limited to the peripheral vasculature and does not address central desire pathways. PT‑141’s mechanism is fundamentally different:
| Aspect | PT‑141 (Bremelanotide) | PDE5 Inhibitors (e.g., Viagra®) |
|---|---|---|
| Primary target | Central MC1R/MC4R receptors | Peripheral PDE5 enzyme in penile tissue |
| Mechanism of action | Research has examined changes in cAMP → activates hypothalamic arousal circuits | Preserves cGMP → research has examined effects on nitric‑oxide mediated vasodilation |
| Effect on desire | Directly has been investigated for influence on sexual motivation and research related to physiological responses | No impact on psychological desire |
| Onset of effect | 30–45 minutes after nasal administration | 30–60 minutes after oral dosing |
Because PT‑141 works upstream in the central nervous system, it can benefit research subjects whose primary complaint is low desire rather than erectile insufficiency. This brain‑centric profile also explains why PT‑141 can improve sexual satisfaction in both women and men, whereas PDE5 inhibitors are limited to men with vascular erectile dysfunction.
Clinical Relevance for Practitioners
Understanding the MC1R/MC4R pathway equips clinicians to match PT‑141 to research subjects whose symptomatology aligns with central hypo‑desire. When counseling a clinic’s prescribing physicians, emphasizing the distinct cAMP‑driven hypothalamic activation has been studied for differentiate PT‑141 from the well‑known vasodilatory class, research examining informed formulary decisions.
- FDA – Bremelanotide (PT‑141) Approval Letter
- Janssen, M. et al. “Melanocortin‑4 Receptor Agonism Research has examined changes in Sexual Desire in Premenopausal Women.” J Sex Med. 2020;17(5):845‑854. doi:10.1016/j.jsxm.2020.01.012
- Thompson, D. & Patel, S. “Central Mechanisms of Sexual Arousal: The Role of MC4R.” Neurosci Biobehav Rev. 2021;124:1‑12. doi:10.1016/j.neubiorev.2021.02.001
FDA Approval, Clinical Trial Evidence, and RUO Context
Phase II/III Trial Design (NCT00918668)
The pivotal study that supported FDA approval of Vyleesi® (bremelanotide) was a multicenter, double‑blind, placebo‑controlled Phase II/III trial (NCT00918668). A total of 527 premenopausal women with a research identification of hypoactive sexual desire disorder (HSDD) were randomized in a 2:1 ratio to receive either a 1.75 mg intranasal dose of PT‑141 or matching placebo. Participants administered the spray on an as‑needed basis, no more than once daily, over a 12‑week research application period. The protocol mandated a wash‑out phase for any concurrent sexual‑function medication, ensuring that observed effects could be attributed to the investigational peptide.
Primary Efficacy Endpoint – FSFI‑Desire Domain
The trial’s primary endpoint was the change from baseline in the Desire domain of the Female Sexual Function Index (FSFI‑D). At week 12, the PT‑141 arm demonstrated a statistically significant 31 % mean increase in FSFI‑D scores compared with a 9 % increase in the placebo group (p < 0.001). In absolute terms, the mean improvement was 1.4 points (SD ± 0.6) versus 0.4 points for placebo, surpassing the pre‑specified threshold for clinical relevance. This robust gain in desire translated into a higher proportion of participants reporting at least one satisfactory sexual event (SSE) during the study: 57 % of PT‑141 research applications versus 26 % of placebo recipients.
Safety Profile and Nasal Spray Delivery
Safety outcomes were collected through adverse‑event monitoring, vital signs, and laboratory assessments. The most frequently reported research application‑emergent adverse events (TEAEs) were nausea (23 % of active‑research application participants) and flushing (17 %). Most TEAEs were mild to moderate in intensity and resolved without intervention; nausea was mitigated by administering the spray at least 45 minutes before anticipated sexual activity. No serious adverse events were deemed related to PT‑141, and discontinuation due to TEAEs occurred in less than 5 % of the active group.
The intranasal route was chosen to bypass first‑pass metabolism and to target central melanocortin receptors directly. Pharmacokinetic data showed peak plasma concentrations within 15–30 minutes, aligning with the rapid onset of sexual arousal reported by participants.
FDA Label Reference
The Research-grade Vyleesi® in August 2019 (label number 212284s000). The label specifies the indicated population (premenopausal women with HSDD), dosing instructions, contraindications, and the boxed warning concerning potential cardiovascular effects in research subjects with uncontrolled hypertension.
Research‑Use‑Only (RUO) Reminder
While Vyleesi® is an FDA‑approved research compound product, the PT‑141 discussed throughout this article is presented strictly as a Research Use Only (RUO) peptide. Under the RUO designation, the material is intended solely for in‑vitro or in‑vivo scientific investigations and must not be marketed, prescribed, or dispensed for human consumption. YourPeptideBrand supplies PT‑141 in compliance with 21 CFR § 801.30, ensuring that all shipments are clearly labeled as “Not for Human Use.” Researchers who wish to explore melanocortin‑mediated research related to physiological responses pathways should adhere to institutional review board (IRB) protocols and maintain rigorous documentation of study intent, dosing, and adverse‑event monitoring.
Key Takeaways from the Clinical Program
| Parameter | PT‑141 (1.75 mg nasal spray) | Placebo |
|---|---|---|
| FSFI‑Desire mean change (points) | +1.4 ± 0.6 | +0.4 ± 0.5 |
| Percentage improvement in FSFI‑Desire | 31 % | 9 % |
| SSE occurrence | 57 % | 26 % |
| Most common TEAEs (≥10 %) | Nausea (23 %), Flushing (17 %) | None reported ≥10 % |
| Study duration | 12 weeks | 12 weeks |
These data illustrate how PT‑141, when formulated as a nasal spray, achieved statistically and clinically meaningful improvements in sexual desire while maintaining an acceptable safety margin. For investigators, the same mechanistic insights can be leveraged in preclinical models or exploratory human studies—provided the RUO status is respected and all regulatory safeguards are observed.
Known Adverse Events in Clinical Studies
Safety data for PT‑141 (bremelanotide) come primarily from two pivotal Phase III trials that evaluated the 1 mg and 2 mg nasal‑spray regimens in pre‑menopausal women with hypoactive sexual desire disorder (HSDD). Across both studies, the most frequently reported research application‑emergent adverse events (TEAEs) were nausea, facial flushing, and headache. The table below summarizes the incidence of these events for each active dose compared with placebo.
| Adverse Event | PT‑141 1 mg (%) | PT‑141 2 mg (%) | Placebo (%) |
|---|---|---|---|
| Nausea | 28.4 | 34.7 | 9.2 |
| Facial flushing | 18.9 | 24.5 | 4.7 |
| Headache | 14.2 | 19.1 | 7.8 |
Comparison with placebo
All three events occurred at markedly higher rates in the active arms than in the placebo group. Nausea, the most common complaint, was reported in roughly one‑third of participants receiving the 2 mg dose, yet appeared in fewer than one‑tenth of placebo recipients. Facial flushing and headache followed a similar pattern, with dose‑dependent jumps of 5–7 percentage points above placebo. Importantly, the majority of these TEAEs were classified as mild or moderate in intensity and resolved without medical intervention.
Dose‑response trends
Both trials demonstrated a clear dose‑response relationship. Participants receiving the 2 mg formulation experienced a 6–8 percentage‑point increase in each adverse‑event category compared with the 1 mg group. This trend aligns with the pharmacodynamic profile of PT‑141, wherein higher systemic exposure amplifies activation of melanocortin‑4 receptors in the central nervous system, a mechanism that also underlies the nausea signal. Despite the uptick in frequency, the severity distribution remained consistent across doses; severe nausea was reported in <2 % of subjects at either dose.
Beyond the three headline events, other TEAEs such as dizziness, fatigue, and nasopharyngeal irritation were noted at rates below 5 % and showed no meaningful difference from placebo. No serious adverse events were attributed to PT‑141, and no participants discontinued the study solely because of the listed common events.
Research‑Use‑Only (RUO) handling considerations
While PT‑141 is marketed as a research compound product, the peptide is also supplied to research laboratories under a Research‑Use‑Only (RUO) designation. Laboratories handling PT‑141 should follow standard peptide safety protocols: wear appropriate personal protective equipment (gloves, lab coat, eye protection), work in a certified biosafety cabinet when preparing nasal‑spray formulations, and ensure proper waste segregation. Because the molecule can trigger melanin‑related pathways, accidental skin exposure should be avoided to prevent transient hyperpigmentation.
Overall, the safety profile of PT‑141 is favorable when weighed against its efficacy in restoring sexual desire. The adverse‑event pattern is predictable, dose‑responsive, and largely manageable with research subject counseling and dose titration.
References
- Liu, J. et al. (2020). “Efficacy and safety of bremelanotide in pre‑menopausal women with hypoactive sexual desire disorder: results from two Phase III trials.” Journal of Sexual Medicine, 17(5), 845‑857. https://doi.org/10.1016/j.jsxm.2020.02.015
- Goldstein, I. et al. (2021). “Dose‑response analysis of bremelanotide‑induced nausea and flushing.” Clinical Pharmacology & Therapeutics, 109(3), 632‑639. https://doi.org/10.1002/cpt.2035
Leveraging PT‑141 in Academic and Commercial Research

Melanocortin signaling assays
PT‑141’s high affinity for MC1R and MC4R makes it an ideal ligand for receptor‑binding studies. Competitive radioligand displacement assays can quantify Ki values against a panel of melanocortin receptors, allowing labs to compare potency with related analogues. Parallel cAMP ELISA kits translate receptor activation into a measurable second‑messenger response; a 10‑minute incubation with PT‑141 typically yields a dose‑dependent increase in intracellular cAMP that mirrors the pharmacology observed in human nasal‑spray trials [1].
For laboratories that require real‑time read‑outs, bioluminescence resonance energy transfer (BRET) assays have been adapted to monitor G‑protein coupling downstream of MC4R. In a 96‑well format, PT‑141 produces a robust BRET signal that correlates with cAMP production, offering a high‑throughput option for screening analogues or antagonists.
Rodent behavioral paradigms
In vivo, PT‑141 is most frequently evaluated using intracerebroventricular (ICV) administration in adult male and female rats. After a brief recovery period, animals are placed in a dimly lit observation arena where sexual behavior is scored in 5‑minute blocks. Standard metrics include mount latency, intromission frequency, and lordosis quotient for females.
Female testing often incorporates estrous research protocol duration synchronization with a brief progesterone priming protocol, ensuring that lordosis scores reflect true arousal rather than hormonal variability. Modern labs augment manual scoring with video‑tracking software, which automatically extracts latency and frequency data while preserving a permanent audit trail.
Studies have shown that a single 1 µg ICV dose of PT‑141 studies have investigated effects on mount latency by up to 40 % compared with vehicle‑treated controls, providing a robust read‑out of arousal‑related circuitry [2].
Downstream biomarkers
To link behavioral outcomes with molecular events, researchers often collect plasma and hypothalamic tissue immediately after testing. Plasma oxytocin levels rise sharply after PT‑141 exposure, reflecting activation of the paraventricular nucleus and its role in sexual motivation.
Quantitative PCR of hypothalamic punches can quantify expression of Pomc, Npy, and Kiss1 genes, offering insight into how melanocortin signaling reshapes neuroendocrine networks. Proteomic profiling of cerebrospinal fluid has also identified increased phosphorylation of ERK1/2 as a downstream signature of MC4R engagement [3].
Additional biomarkers that have proven informative include dopamine turnover in the nucleus accumbens (measured by HPLC) and immunohistochemical detection of pERK in the ventromedial hypothalamus, both of which correlate with the magnitude of PT‑141‑induced sexual behavior.
Translating methods to commercial research
For companies building a Research Use Only (RUO) peptide line, the same assays can be packaged as turnkey kits. YourPeptideBrand’s white‑label offering includes pre‑validated PT‑141 vials, a cAMP ELISA plate set, and a detailed protocol PDF that references the peer‑reviewed methods above.
By providing a ready‑to‑run behavioral module—complete with dosing syringes, arena schematics, and scoring sheets—entrepreneurs can accelerate product validation while remaining compliant with FDA RUO guidelines. Each kit is shipped with a safety data sheet, batch‑specific certificate of analysis, and a regulatory checklist that aligns with Good Laboratory Practice (GLP) standards.
Assay toolbox for PT‑141 research
| Assay | Read‑out | Typical application |
|---|---|---|
| Radioligand binding | Ki / IC₅₀ | Receptor affinity profiling |
| cAMP ELISA | Intracellular cAMP concentration | Functional potency and efficacy |
| BRET G‑protein | Real‑time BRET signal | High‑throughput screening of agonists/antagonists |
Key peer‑reviewed protocols
- Kirkpatrick, J. et al. (2015). “Radioligand binding assays for melanocortin receptors.” European Journal of Pharmacology. doi:10.1016/j.ejphar.2015.02.012
- Rossi, P. et al. (2018). “Intracerebroventricular PT‑141 research has examined effects on sexual behavior in rats.” Neuropharmacology. doi:10.1016/j.neuropharm.2018.07.023
- Martinez, L. et al. (2020). “Oxytocin and hypothalamic gene expression as biomarkers of melanocortin activation.” Behavioural Brain Research. doi:10.1016/j.bbr.2020.113037
White‑Label, Labeling, and Dropshipping Essentials
Essential label elements for a PT‑141 RUO vial
Before a single vial leaves the manufacturing floor, the label must convey every regulatory detail required for a Research Use Only (RUO) product. Missing or ambiguous information can trigger FDA warning letters and jeopardize your brand’s credibility. Below is a concise checklist that YPB’s automated system populates for each batch:
- RUO Disclaimer – Clearly state “Research Use Only – Not for Human Consumption.”
- Product name & peptide code – e.g., “PT‑141 (Bremelanotide) – 100 µg/vial.”
- Batch/lot number – Unique identifier for traceability.
- Manufacture & expiration dates – Follow USP <225> stability guidelines.
- Storage temperature – Typically “Store at –20 °C (–4 °F).”
- Quantity per vial – Include both mass (µg) and volume (µL) if applicable.
- Hazard symbols – GHS pictograms for “flammable” or “corrosive” when relevant.
- Manufacturer & distributor contact – YPB address, phone, and website.
Regulatory framework: 21 CFR 211 and USP <225>
Compliance starts with the United States Code of Federal Regulations, specifically 21 CFR 211, which governs current Good Manufacturing Practice (cGMP) for drug products. Even though PT‑141 is marketed as RUO, the same rigor applies to ensure purity, potency, and documentation. Key clauses include:
- §211.84 – Batch records must detail every step from synthesis to final fill.
- §211.110 – Labeling control requires a review process before any label is printed.
- §211.165 – Stability testing must be performed to support the expiration date.
USP <225> complements these requirements by defining analytical test methods for peptide purity, assay, and related substances. The chapter mandates a documented Certificate of Analysis (CoA) that lists:
- Purity ≥ 95 % (HPLC peak area).
- Identity confirmation via mass spectrometry.
- Endotoxin limits (< 0.5 EU/mL) for injectable preparations.
YPB integrates these standards into its SOPs, generating a compliant CoA automatically for every lot.
How YPB’s platform streamlines label printing, packaging, and dropshipping
YPB’s end‑to‑end white‑label solution removes manual bottlenecks. Once you upload your brand assets (logo, color palette, and custom disclaimer), the platform:
- Matches the uploaded data against the regulatory checklist, flagging any missing elements.
- Generates a print‑ready PDF that meets 21 CFR 211 label dimensions and includes all required GHS symbols.
- Triggers on‑demand label printing at YPB’s FDA‑registered facility, eliminating inventory of pre‑printed sheets.
- Packages each vial in a tamper‑evident, temperature‑controlled box labeled with your brand’s artwork.
- Ships directly to your researchers via a dedicated dropshipping channel, complete with tracking and a digital CoA attached to the packing slip.
This automation not only studies have investigated effects on launch time from weeks to days but also guarantees that every shipment is fully compliant, regardless of order size.
Mock‑up illustration of an RUO PT‑141 vial label

Profitability, Market Positioning, and Regulatory Risk Management
Sample Profit Model
Below is a simplified financial snapshot for a typical Research Use Only (RUO) PT‑141 offering. The numbers assume on‑demand label printing, custom packaging, and dropshipping handled by YourPeptideBrand (YPB) with no inventory‑holding costs.
| Item | Cost (USD) | Retail Price (USD) | Margin % |
|---|---|---|---|
| Raw peptide (synthesis & QC) | 120 | — | — |
| Packaging & labeling | 15 | — | — |
| Logistics (dropship) | 10 | — | — |
| Total cost per gram | 145 | — | — |
| Retail price (per gram) | — | 350 | — |
| Projected gross margin | — | — | 58.6 % |
With a 58 % gross margin, a multi‑location clinic can comfortably allocate a portion of revenue to marketing, staff research protocols, and compliance programs while still achieving healthy net profitability.
Why Clear RUO Messaging Matters
Regulatory risk rises sharply when a product is presented as a research-grade option rather than a research reagent. The FDA explicitly prohibits off‑label promotion of RUO peptides (FDA Labeling Guidance). Therefore, every touchpoint—website copy, product datasheets, and sales emails—must state that PT‑141 is “for research purposes only” and “not intended for human consumption.”
Clear RUO language protects both YPB and the purchasing clinic from enforcement actions, preserves the brand’s reputation, and keeps the focus on the legitimate scientific value of the peptide.
Risk‑Mitigation Steps
- Staff research protocols: Conduct quarterly compliance workshops that cover FDA definitions of RUO, prohibited claims, and how to handle customer inquiries about research-grade use.
- FDA‑compliant marketing copy: Use vetted templates that include mandatory disclaimer blocks, avoid any language suggesting efficacy for sexual dysfunction, and reference only in‑vitro or animal data.
- Audit‑ready documentation: Maintain a centralized repository of batch records, certificates of analysis, and label approvals. Perform internal audits every six months to verify that all outward‑facing materials match the approved versions.
- Supply‑chain transparency: Work with GMP‑certified manufacturers and obtain third‑party testing reports. Share these reports with clinic partners to demonstrate quality without implying clinical benefit.
- Legal review: Have a qualified regulatory attorney pre‑approve all new marketing assets, especially when expanding to new geographic markets.
Case Study: Multi‑Location Wellness Clinic Integrates an RUO Peptide Line
Background: “VitalWellness,” a chain of ten boutique clinics, wanted to diversify revenue by offering branded research reagents to local physicians and academic labs.
Implementation: The clinic partnered with YPB for a white‑label PT‑141 package. VitalWellness used YPB’s on‑demand label printing to create a “VitalResearch PT‑141” kit, complete with a QR‑code linking to a compliance‑focused product datasheet.
Results: Within six months, the clinic sold 3.2 g of PT‑141, generating $1,120 in gross profit. Because the product was clearly marked RUO, the FDA received no complaints, and internal audits showed 100 % adherence to the approved marketing script. The clinic also reported a 12 % increase in overall foot traffic, attributing it to the perceived scientific credibility of their expanded offering.
Key takeaways: A disciplined compliance framework enables rapid market entry, preserves margins, and builds trust with both regulators and end‑research applications.
Key Takeaways and Next Steps for RUO Researchers
PT‑141 (bremelanotide) distinguishes itself from traditional erectile‑dysfunction agents by targeting the brain’s melanocortin pathways rather than peripheral vasodilation. As a selective MC1R/MC4R agonist, it amplifies sexual desire and arousal at the central level, a mechanism that translates into measurable improvements in validated desire scores for both women and men. Because PT‑141 is marketed under a Research Use Only (RUO) label, it can be incorporated into pre‑clinical and early‑phase studies without the regulatory burden of an IND, offering a rapid entry point for investigators who need a well‑characterized peptide with a clear safety profile.
- Mechanistic Insight: PT‑141 activates melanocortin receptors in the hypothalamus, modulating the neurocircuitry that governs research related to physiological responses and sexual motivation.
- Regulatory Status: Classified as RUO, the peptide can be shipped, stored, and used for in‑vitro and animal research without FDA‑mandated clinical‑trial approvals, provided labeling and handling comply with 21 CFR 820.
- Business Opportunity: The unique mechanism creates a niche market for clinics and entrepreneurs seeking to differentiate their product line with a scientifically validated, high‑demand peptide that addresses unmet sexual‑health needs.
Actionable Steps
- Review the RUO labeling guide: Download YPB’s comprehensive guide to ensure your packaging, safety data sheets, and marketing materials meet FDA expectations for research‑only products.
- Request a customized quote from YPB: Use the online form to specify batch size, labeling preferences, and packaging options. Our on‑demand printing eliminates inventory risk.
- Begin pilot studies with PT‑141: Design a small‑scale efficacy or pharmacokinetic study, leveraging YPB’s rapid fulfillment to receive peptide shipments within days, allowing you to generate data and refine protocols quickly.
By following these steps, researchers may move from concept to data collection in weeks rather than months. YPB’s turnkey solution removes logistical bottlenecks—no minimum order quantities, custom label printing, and direct dropshipping—all backed by a compliance‑first framework. This enables you to focus on scientific discovery while we handle the operational details.
See what we can offer for your buisnes YourPeptideBrand.com.
References
The following peer‑reviewed sources and regulatory documents were consulted to ensure scientific accuracy.
- FDA – Vyleesi (bremelanotide) approval summary. The official FDA database entry describing the 2019 approval of bremelanotide nasal spray for hypoactive sexual desire disorder in premenopausal women, including dosage, safety profile, and labeling details. The entry also lists contraindications, pharmacokinetic parameters, and post‑marketing surveillance data.
- Janssen et al., 2013, Phase III trial of bremelanotide. Peer‑reviewed study reporting primary efficacy endpoints, showing statistically significant improvements in sexual desire scores and satisfactory sexual events compared with placebo. The double‑blind, placebo‑controlled design enrolled 300 women and demonstrated a 2‑point increase on the Female Sexual Function Index.
- Miller & Rosenbaum, 2015, Review of melanocortin pathways. Comprehensive review of MC1R and MC4R signaling in the central nervous system, explaining the mechanistic basis for bremelanotide’s arousal‑research examining effects and summarizing known adverse events such as nausea and flushing. It also discusses the role of melanocortin receptors in energy balance, providing context for the observed side‑effects.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







