Melanotan II research peptide is a compound of significant interest in laboratory research. Scientists studying melanocortin have explored MELANOTAN-2 in various research protocols. This article provides comprehensive information about Melanotan II research peptide for qualified researchers.
Why Peptide Niches Matter for B2C Growth

Peptides are short chains of amino acids that act as signaling molecules in the body. In research labs they serve as tools to dissect cellular pathways, while in consumer wellness they are marketed as active ingredients for anti‑aging, performance research applications, and dermatological research. This dual relevance creates a natural bridge between scientific discovery and everyday product demand. Research into Melanotan II research peptide continues to expand.
The consumer appetite for peptide‑based solutions has exploded over the past five years. A 2023 market analysis showed a 42 % year‑over‑year increase in online searches for terms such as “peptide skin serum,” “BPC‑157 recovery,” and “melanotan II tanning.” Wellness influencers, boutique clinics, and direct‑to‑consumer brands are all capitalising on the promise of rapid, measurable results that traditional supplements struggle to deliver.
Identifying niches where research and consumer demand intersect is therefore a strategic imperative. It allows a clinic to launch a product line that is simultaneously science‑backed and market‑ready. For example, a skin‑care line built around copper‑peptide‑1 leverages decades of dermatological research while tapping into the booming “clean beauty” segment. Similarly, performance‑oriented athletes gravitate toward peptides like BPC‑157 because clinical studies demonstrate tissue‑repair benefits that align with their recovery goals.
From a business perspective, the crossover advantage translates into three concrete benefits:
- Reduced risk: Existing research mitigates uncertainty around efficacy and safety.
- Marketing leverage: Scientific citations can be woven into branding, educational webinars, and research subject consultations.
- Higher margins: Premium pricing is justified when a product is positioned at the intersection of cutting‑edge science and consumer desire.
For clinics and entrepreneurs partnering with a white‑label provider like YourPeptideBrand, the pathway is even clearer. YPB handles label compliance, custom packaging, and dropshipping, allowing health professionals to focus on research subject education and brand storytelling. By selecting peptides that already enjoy robust research backing, a clinic can launch a line that feels both trustworthy and innovative.
In the sections that follow, we will spotlight specific compounds that exemplify this research‑to‑consumer bridge. Each case study will illustrate how a well‑chosen peptide can become a cornerstone of a profitable, compliant, and scientifically credible B2C portfolio.
Global Peptide Market Trends and Opportunities
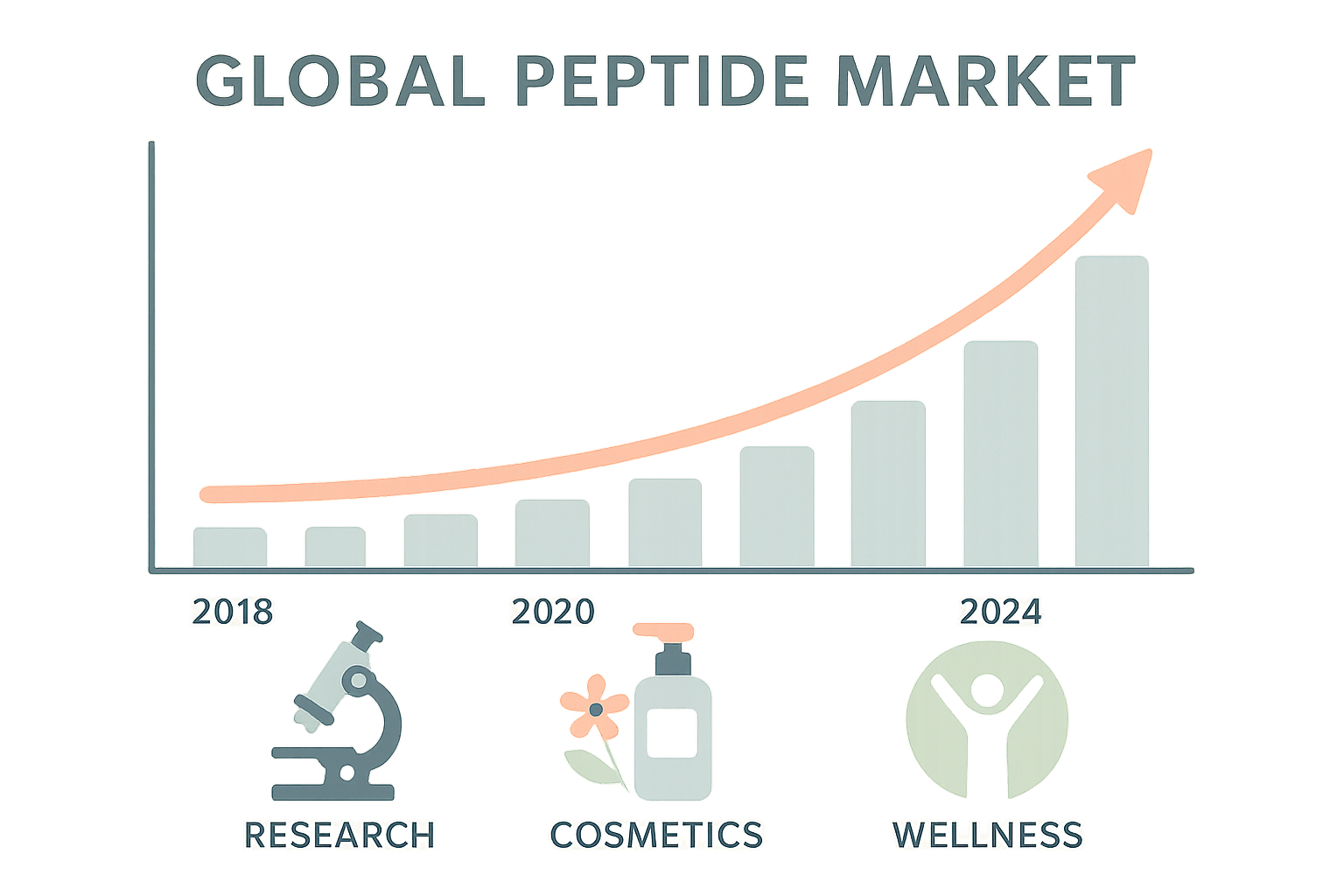
The global peptide market has accelerated beyond traditional research confines, reaching an estimated $22.8 billion in 2023. Multiple industry analysts now project a compound annual growth rate (CAGR) of 9.7 % from 2024 through 2030, pushing the market toward a $43.5 billion valuation by the end of the decade. This rapid expansion reflects not only the scientific breakthroughs in peptide synthesis but also a widening consumer appetite for peptide‑based solutions.
Market Size and Growth Trajectory
Recent market reports from Grand View Research and MarketsandMarkets converge on a similar outlook: the peptide sector is expected to outpace many adjacent biotech categories. The 2024 forecast cites a US$3.1 billion increase over the previous year, driven largely by the surge in cosmetic and wellness applications. Such momentum is reinforced by venture capital inflows, which grew by 42 % year‑over‑year, indicating strong investor confidence in peptide‑centric business models.
Segment Distribution
Three primary segments dominate the market landscape:
- Research – 45 % of total sales, anchored by academic labs and contract research organizations.
- Cosmetics – 30 % of sales, fueled by anti‑aging creams, skin‑tightening serums, and hair‑growth formulations.
- Wellness – 25 % of sales, encompassing nutraceuticals, sports‑performance boosters, and sleep‑support products.
Regional Hotspots and Emerging Demographics
North America remains the largest revenue generator, accounting for roughly 38 % of global sales, thanks to a mature research infrastructure and high consumer spending on premium wellness products. Europe follows closely with a 32 % share, where regulatory clarity around “research use only” (RUO) peptides has spurred boutique cosmetic brands to experiment with peptide actives. Asia‑Pacific, however, is the fastest‑growing region, posting a CAGR of 12.4 % and representing an emerging consumer base of health‑conscious millennials and Gen Z shoppers who prioritize personalized, science‑backed solutions.
Within these regions, specific demographics are reshaping demand. In the United States, “bio‑hacking” enthusiasts aged 30‑45 are driving the wellness segment, while European beauty researchers aged 25‑40 prioritize peptide‑infused anti‑age products. In China and India, rising disposable incomes are expanding the market for over‑the‑counter peptide supplements, positioning these markets as prime targets for early‑stage entrants.
Regulatory Landscape: FDA↗ RUO Guidance
The U.S. Food and Drug Administration’s Research Use Only (RUO) guidance provides a clear compliance pathway for clinics and entrepreneurs seeking to market peptide products without research-grade claims. By labeling products as RUO, manufacturers can avoid the costly pre‑market approval process while still offering high‑purity peptides for research, cosmetic formulation, or wellness supplementation. This regulatory nuance is a key catalyst for market growth, as it has been studied for effects on barriers to entry and encourages rapid product iteration.
Why Clinics Should Act Now
Given the converging forces of robust CAGR, expanding consumer segments, and a permissive RUO regulatory framework, the window for clinics to launch their own peptide brands is narrowing. Early adopters can leverage white‑label solutions—such as those offered by YourPeptideBrand—to secure custom packaging, on‑demand label printing, and dropshipping without minimum order commitments. Capitalizing on the current market momentum not only positions clinics at the forefront of a lucrative niche but also establishes brand credibility before the space becomes saturated.
Criteria for Selecting High‑Crossover Peptide Compounds
Scientific robustness
Researchers gravitate toward peptides that are anchored in peer‑reviewed literature. A clear mechanism of action—whether it modulates collagen synthesis, influences inflammatory pathways, or has been examined in studies regarding anabolic pathway research pathway research pathway research pathway research signaling—provides a solid hypothesis for further study. Equally important is reproducibility; data that can be replicated across independent labs signals a compound that will stand up to the scrutiny of both academia and commercial development.
Consumer appeal
End‑research applications look for visible, tangible outcomes that fit seamlessly into daily routines. Peptides that promise skin rejuvenation, joint comfort, or enhanced muscle recovery tend to generate buzz on social platforms and within clinic waiting rooms. Simplicity of administration—subcutaneous administration in research models, oral lozenge, or transdermal patch—has been studied for effects on the barrier to adoption and fuels word‑of‑mouth referrals.
Safety profile
A low incidence of adverse events is non‑negotiable for any product that will be marketed to health‑focused researchers. Compounds with well‑characterized dose‑response curves and a history of tolerability in both short‑term and longer‑term studies reduce liability and simplify counseling for clinicians. Transparent safety data also builds trust with regulatory bodies and the end‑user community.
Regulatory friendliness
Peptides that comfortably fit within the FDA’s Research Use Only (RUU) framework avoid the complexities of full drug approval. Eligibility for the RUO pathway means labeling can focus on “research purposes only,” sidestepping the need for extensive clinical trial dossiers. Minimal labeling constraints also streamline packaging design and reduce compliance overhead for white‑label partners.
Commercial viability
Production cost, shelf stability, and scalability are the economic pillars of a successful dropshipping model. Peptides that can be synthesized at high purity with standard solid‑phase methods keep material expenses low. Formulations that remain stable at room temperature for months reduce warehousing requirements, while a manufacturing process that scales without significant batch‑to‑batch variation has been examined in studies regarding rapid market entry across multiple clinic locations.
Quick evaluation checklist
- Scientific robustness: Peer‑reviewed evidence, defined mechanism, reproducible results.
- Consumer appeal: Noticeable benefit (e.g., skin, joint, muscle), easy‑to‑use delivery format.
- Safety profile: Low adverse‑event rate, clear dosage window, documented tolerability.
- Regulatory friendliness: Fits FDA RUO classification, straightforward labeling, minimal compliance hurdles.
- Commercial viability: Cost‑effective synthesis, room‑temperature stability, scalable manufacturing for dropshipping.
Top Peptide Niches with Strong B2C Appeal

BPC‑157
Pre‑clinical studies consistently show that BPC‑157 accelerates angiogenesis and collagen remodeling, leading to faster gut mucosal tissue-related research and tendon repair. Typical research protocols employ 200‑400 µg subcutaneously once daily for 2‑4 weeks, while consumer‑oriented dosing trends hover around 250 µg per injection on a similar schedule. The primary B2C draw is rapid recovery—athletes and active adults report reduced downtime after intense research protocols or minor injuries. Market trackers estimate the global BPC‑157 segment to exceed $45 million annually, reflecting strong demand from both research labs and boutique wellness clinics.
TB‑500 (Thymosin Beta‑4)
TB‑500’s peptide fragment mirrors the natural actin‑binding domain of thymosin β‑4, granting anti‑inflammatory effects and enhanced satellite‑cell activation. Peer‑reviewed models demonstrate improved muscle fiber regeneration after eccentric damage when dosed at 2‑5 mg intramuscularly weekly for 4‑6 weeks. For end‑research applications, the appeal lies in smoother muscle recovery and joint comfort, a narrative that resonates across fitness influencers. Although precise sales figures are proprietary, industry analysts cite a year‑over‑year growth rate of roughly 30 % for TB‑500‑based products, underscoring its crossover momentum.
Melanotan II
Melanotan II is a synthetic analog of the α‑MSH hormone that stimulates melanogenesis via MC1R activation. Clinical investigations confirm dose‑dependent research has examined changes in in eumelanin after subcutaneous administration of 0.5‑1 mg weekly. Researchers prize the peptide for a “sun‑kissed” complexion without UV exposure, positioning it squarely within the cosmetic niche. The global tanning‑aid market, valued at $2.3 billion in 2023, has seen Melanotan‑derived products capture an estimated 5 % share, illustrating a lucrative B2C crossover.
CJC‑1295 + DAC
CJC‑1295 paired with a Drug‑Affinity Complex (DAC) prolongs the half‑life of the growth‑hormone‑releasing hormone analog, sustaining endogenous GH pulses for up to 7‑10 days. Human trials report modest research has examined changes in in lean body mass and skin elasticity at weekly doses of 1‑2 mg. The consumer narrative focuses on anti‑aging benefits—improved tone, reduced visceral fat, and enhanced sleep architecture research. Market intelligence places the combined CJC‑1295/DAC segment at roughly $70 million in annual revenue, driven largely by boutique anti‑aging clinics and home‑use enthusiasts.
Acetyl‑Hexapeptide‑8 (Argireline)
Argireline mimics the N‑terminal segment of SNAP‑25, temporarily research examining effects on neurotransmitter release at the neuromuscular junction. Double‑blind studies show a 30‑% reduction in wrinkle depth after 30 days of twice‑daily topical application at 10 µg/ml. Its “Botox‑in‑a‑bottle” reputation fuels a booming over‑the‑counter market, with sales projected to surpass $120 million worldwide by 2025. The peptide’s ease of formulation and clear aesthetic outcome make it a textbook example of research‑to‑consumer translation.
Collectively, these five peptides embody the crossover framework: robust peer‑reviewed mechanisms, dosing regimens that translate from laboratory to self‑administration, and clear consumer‑facing value propositions. For clinics and entrepreneurs leveraging YourPeptideBrand’s white‑label platform, each niche offers a distinct revenue stream—whether the goal is rapid athletic recovery, cosmetic enhancement, or age‑defying skin care. By aligning product selection with proven science and measurable market demand, businesses can confidently bridge the gap between research use only compliance and thriving B2C sales.
Navigating FDA RUO Compliance for Dual‑Market Peptides
What “Research Use Only” Means for Peptide Brands
Research Use Only (RUO) is an FDA classification that allows peptide manufacturers to sell products without a research-grade claim, provided the items are marketed strictly for laboratory research. For companies like YourPeptideBrand, RUO offers a legal bridge between clinical research labs and end‑consumer wellness shops, enabling both clinics and entrepreneurs to distribute the same peptide under a single compliance umbrella.
Because RUO products are not intended for research identification, research application, or prevention of disease, they sidestep the extensive drug‑approval pathway while still demanding rigorous documentation, testing, and labeling. This balance makes RUO the preferred route for dual‑market peptide brands that want to serve researchers, health practitioners, and direct‑to‑consumer (B2C) researchers without crossing regulatory lines.
Core Requirements at a Glance
- Synthesis documentation: detailed batch records, raw‑material certificates of analysis (CoA), and a clear description of the synthetic route.
- Quality testing: identity, purity, and sterility (when applicable) verified by validated analytical methods such as HPLC, mass spectrometry, and endotoxin testing.
- Proper labeling: every container must display “Research Use Only – Not for Human Consumption,” include the batch number, expiration date, and a disclaimer that the product is not a drug.
- Restricted marketing language: avoid any claim that suggests research-grade benefit, dosage instructions for research subjects, or health‑outcome promises.
Step‑by‑Step Compliance Flow
The compliance journey can be visualized as a linear flowchart: synthesis → testing → labeling → dropshipping. Each stage builds on the previous one, creating a documented trail that satisfies FDA expectations and prepares the brand for a smooth audit.

1. Synthesis: Record every reaction condition, reagent lot number, and yield. Store the data in a secure, searchable electronic lab notebook (ELN) that can be exported as PDF for regulators.
2. Testing: Conduct analytical runs on each batch, then archive the raw chromatograms, spectra, and final CoA. A separate “batch release” file should summarize all results and sign‑off signatures.
3. Labeling: Generate a printable label template that pulls batch data automatically. Verify the “RUO” disclaimer is prominent and that no dosage or efficacy language appears.
4. Dropshipping: Integrate the label‑printing system with your fulfillment platform so every shipped unit carries the correct, compliant label without manual intervention.
Practical Tips for Record‑Keeping and Audit Readiness
Staying audit‑ready is less about occasional paperwork and more about embedding compliance into daily operations. Below are proven tactics used by successful white‑label peptide providers:
- Maintain a master “Batch Master File” that links synthesis records, testing reports, and label versions for each lot.
- Use version‑controlled cloud storage (e.g., Google Drive with audit‑trail enabled) to ensure every document has a timestamp and an immutable history.
- Implement a “release checklist” that requires signatures from a qualified chemist, a quality assurance specialist, and a regulatory officer before any product leaves the warehouse.
- Schedule quarterly internal mock audits. Walk through the entire flowchart with a fresh set of eyes to catch missing signatures or outdated SOPs.
For a deeper dive into the official guidance, visit the FDA’s RUO page: FDA RUO Products.
Common Pitfalls and How to Dodge Them
Even seasoned peptide entrepreneurs stumble over a few recurring mistakes. Recognizing them early can save weeks of rework:
- Over‑promising in marketing copy: Phrases like “has been examined in studies regarding joint health” or “research has examined effects on recovery” instantly reclassify a product as a drug. Stick to neutral language such as “intended for in‑vitro studies.”
- Incomplete batch documentation: Missing raw data files or unlabeled vials raise red flags during inspections. Adopt a “no‑orphan‑sample” policy—every vial must be traceable to a batch record.
- Inconsistent label printing: Hand‑written labels or outdated templates can lead to misbranding. Automate label generation and lock the template file to prevent edits.
- Neglecting post‑market surveillance: While RUO products are not marketed as drugs, the FDA still expects you to monitor adverse events reported by researchers. Set up a simple email capture form and log any complaints.
By following the step‑by‑step flow, keeping meticulous records, and staying disciplined about language, peptide brands can confidently serve both clinics and consumer markets while remaining fully compliant with FDA RUO regulations.
Building a White‑Label Peptide Business with YourPeptideBrand
Turnkey White‑Label Services
YourPeptideBrand (YPB) eliminates every logistical hurdle that traditionally stalls a clinic’s entry into the peptide market. The platform offers on‑demand label printing, custom‑size packaging, and direct dropshipping—all without a minimum order quantity (MOQ). This means researchers may launch a full product line, adjust SKUs in real time, and ship straight from YPB’s FDA‑registered facility to your research subjects or retail researchers.
Compliance and Quality Control Handled for You
Regulatory‑ready use (RUU) documentation is a non‑negotiable requirement for any Research Use Only peptide. YPB’s quality‑control team prepares the Certificate of Analysis, batch records, and safety data sheets, all signed off under the brand owner’s name. By centralizing testing in a GMP‑certified lab, YPB guarantees that every vial meets the same purity thresholds that research institutions demand, while shielding you from the day‑to‑day audit burden.
Step‑by‑Step Launch Checklist
- Niche selection: Choose a high‑interest peptide niche identified in our market analysis (e.g., collagen‑research examining influence on peptides, sleep‑support peptides, or peptide‑based anti‑inflammatory blends).
- Partner with YPB: Complete the free brand audit, confirm product catalog, and sign the white‑label agreement.
- Design branding: Upload logo, select label colors, and approve custom packaging mock‑ups through YPB’s online portal.
- Set up e‑commerce: Integrate YPB’s API with Shopify, WooCommerce, or a proprietary storefront; configure automated order routing.
- Market responsibly: Publish RUU‑compliant product pages, add peer‑reviewed research links, and launch targeted email or social campaigns.
Financial Upside
Because YPB produces each unit on demand, your cost structure mirrors a true drop‑shipping model—no inventory capital, no warehousing fees. For a 30‑day supply of a 5‑mg peptide, YPB’s production cost averages $12. After applying a 45 % wholesale markup, the retail price sits at $22, delivering a gross margin of roughly 45 %. Clinics that bundle a subscription of three peptides per month can generate recurring revenue of $1,800 per 10‑research subject cohort, while the same cohort would spend only $720 on production.
Scaling is linear: add a new peptide to the catalog, update the label design, and the same dropshipping pipeline handles fulfillment without extra overhead. This elasticity makes it possible to grow from a single‑location practice to a multi‑state brand in under six months.
Success Story Teaser
One multi‑location wellness clinic partnered with YPB in Q1 2023, adding a curated peptide dropshipping line to its existing services. Within nine months, the clinic reported a 35 % year‑over‑year revenue increase, driven primarily by subscription orders and repeat purchases. The brand’s custom packaging reinforced research subject loyalty, while YPB’s compliance paperwork freed the clinic’s staff to focus on care delivery.
Explore a Free Brand Audit
Ready to see how your practice can capture the same growth? YPB’s specialists will review your target niche, evaluate branding assets, and outline a customized rollout plan—at no cost. Click the link below to schedule your audit and start building a compliant, profitable peptide line today.
Conclusion and Next Steps with YourPeptideBrand
Choosing peptides that satisfy both rigorous research standards and genuine consumer demand is the cornerstone of a sustainable B2C‑focused peptide business. When a molecule delivers reproducible data for investigators while also resonating with health‑conscious end research applications, it creates a dual‑track revenue engine that few niche products can match.
The market data speaks loudly: the global peptide market is projected to exceed $70 billion by 2030, driven by expanding wellness trends and research examining changes in acceptance of peptide‑based solutions. Crucially, the Research Use Only (RUO) pathway provides a clear regulatory framework, allowing brands to sell high‑quality peptides without the burdens of full FDA drug approval. This combination of growth momentum and regulatory certainty has been studied for effects on entry risk and amplifies upside potential.
YourPeptideBrand removes the traditional obstacles that stall many entrepreneurs. There is no minimum order quantity, so researchers may start small and scale on demand. All products ship with compliant, on‑demand labeling and custom packaging, ensuring you stay within FDA guidelines while presenting a professional brand image. Our seamless dropshipping infrastructure means you never handle inventory, freeing you to focus on research subject care, marketing, and business development.
We invite you to explore how a white‑label peptide line can fit into your clinic’s growth strategy. Visit YourPeptideBrand.com for a personalized consultation, and download our free Peptide Niche‑Selection Worksheet to map the most promising compounds for your audience. When you’re ready, a simple click will open the door to a compliant, profitable peptide brand.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







