Introduction – LL‑37 as a Dual‑Action Human Cathelicidin
The cathelicidin family comprises a group of cationic peptides that serve as front‑line defenders across many species. Humans, however, possess a single member: LL‑37, a 37‑amino‑acid fragment cleaved from the precursor hCAP‑18. First described in 1995, LL‑37 has become the archetype for “host‑derived” antimicrobial peptides.
What sets LL‑37 apart is its two‑pronged activity. On the microbial side, the peptide inserts into bacterial, fungal, and viral membranes, causing rapid disruption and cell death. Simultaneously, LL‑37 acts as a signaling hub for wound repair—stimulating angiogenesis, recruiting fibroblasts, and modulating cytokine release to keep inflammation in check. This duality underpins its reputation as a “master antimicrobial peptide” with intrinsic tissue‑tissue-related research properties. Research into LL-37 research peptide continues to expand.
This guide is crafted for clinicians, clinic owners, and health‑tech entrepreneurs who are evaluating LL‑37 for a Research Use Only (RUO) product line. We emphasize compliance with FDA↗ RUO regulations while outlining commercial opportunities for white‑label, turnkey peptide solutions. Research into LL-37 research peptide continues to expand.
- Detailed molecular architecture and structural dynamics of LL‑37.
- Mechanistic insights into its microbicidal and immunomodulatory actions.
- Regulatory considerations for RUO positioning and pathways to market.
- Business models, pricing strategies, and scalability options for launching a branded peptide line.
Molecular Overview of LL‑37

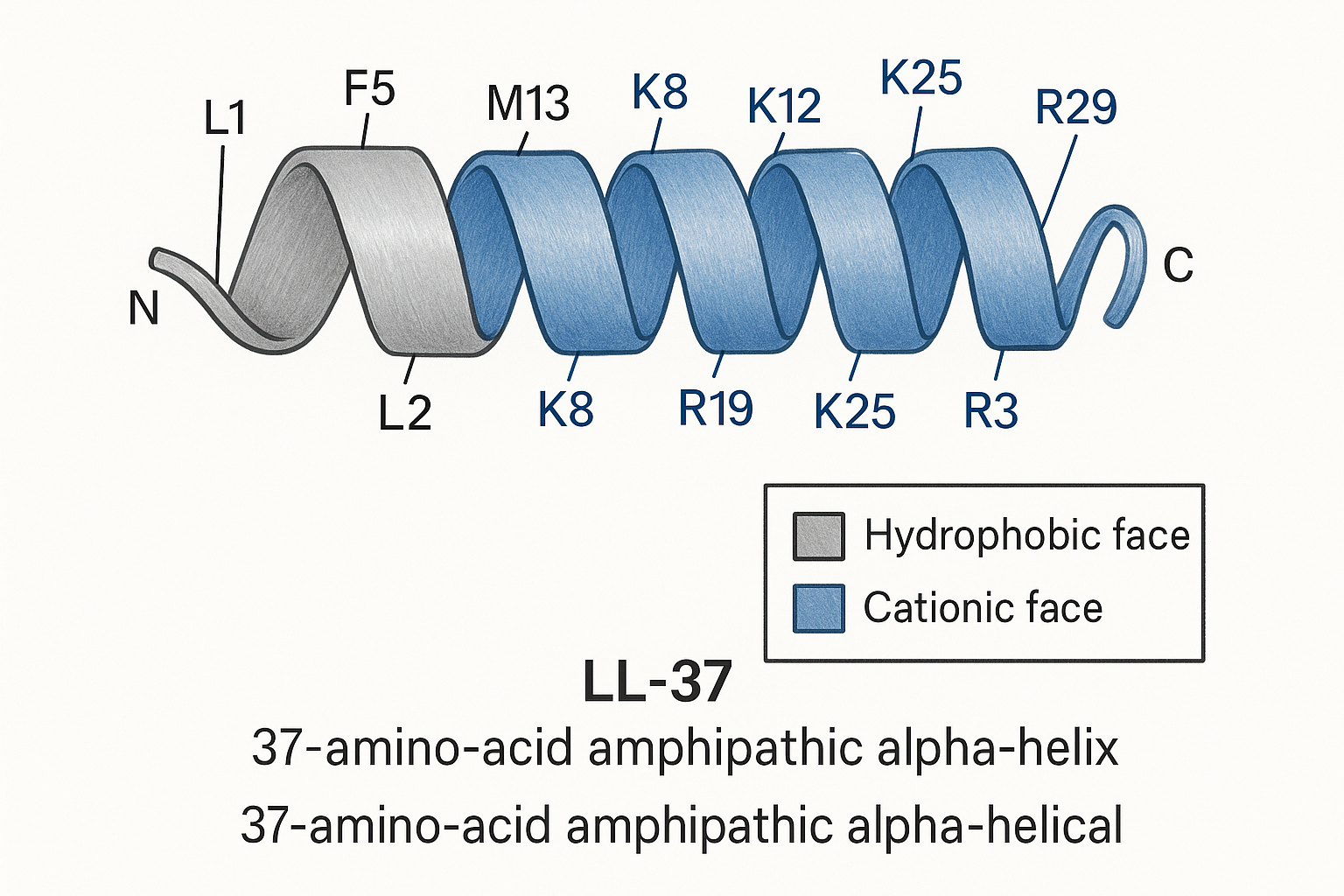
LL‑37 is a 37‑residue, +6‑charged peptide that represents the only human cathelicidin. It originates from the larger precursor protein CAP‑18 (≈170 aa). In neutrophils, proteinase‑3 cleaves CAP‑18 at the C‑terminus, releasing the mature LL‑37 fragment that circulates in plasma and skin.
Primary Sequence and Biosynthetic Origin
The mature peptide sequence is:
LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES
This exact sequence is cataloged in the NCBI RefSeq database (NCBI RefSeq NP_001009), confirming its genetic provenance and enabling reproducible synthesis. Research into LL-37 research peptide continues to expand.
Amphipathic α‑Helical Architecture
When dissolved in aqueous solution, LL‑37 adopts a left‑handed α‑helix that displays a classic amphipathic pattern: one side of the helix is dominated by non‑polar residues (visualized as gray in structural models), while the opposite side clusters basic residues, giving a blue‑colored cationic face. This segregation creates a distinct hydrophobic–hydrophilic interface crucial for membrane interaction. Research into LL-37 research peptide continues to expand.
Mechanism of Membrane Disruption
The amphipathic helix inserts into microbial lipid bilayers by aligning its hydrophobic face with the fatty‑acid core and positioning the positively charged side toward negatively charged phospholipid head groups. This dual affinity drives rapid insertion, destabilization, and formation of transient pores that compromise bacterial, fungal, or viral membranes while sparing most mammalian cells, which lack the requisite surface charge.
Quality Standards and USP <1225>
For research‑use‑only peptide supplies, compliance with USP <1225>—the monograph governing peptide purity testing—is essential. The standard mandates identity confirmation (mass spectrometry), purity assessment (reversed‑phase HPLC), and peptide‑related impurity profiling. Adhering to USP <1225> ensures that LL‑37 batches used in pre‑clinical studies meet rigorous, reproducible quality benchmarks, research examining reliable data generation for clinicians and entrepreneurs alike.
Antimicrobial Mechanisms of LL‑37
Membrane insertion models
LL‑37 attacks microbes by inserting into the lipid bilayer and forming disruptive pores. Two classic models describe this process. In the “carpet” model, peptide molecules coat the surface like a carpet, destabilizing the membrane until it fragments. In the “barrel‑stave” model, α‑helical LL‑37 aligns side‑by‑side, creating a transmembrane channel that allows uncontrolled ion flux and rapid lysis. Both pathways culminate in loss of membrane integrity and cell death within minutes.
Spectrum of activity
The peptide exhibits broad‑range activity against:
- Gram‑positive bacteria (e.g., Staphylococcus aureus, Enterococcus faecalis)
- Gram‑negative bacteria (e.g., Escherichia coli, Pseudomonas aeruginosa)
- Fungi such as Candida albicans and Aspergillus fumigatus
- Enveloped viruses, including influenza A and herpes simplex virus
In‑vitro MIC evidence
Peer‑reviewed studies provide quantitative minimum inhibitory concentration (MIC) values that illustrate LL‑37’s potency. In a 2020 MDPI investigation, the MIC for methicillin‑sensitive S. aureus ATCC 25923 was 4 µg mL⁻¹, while the same peptide inhibited methicillin‑resistant strains at 8 µg mL⁻¹ [1]. A separate 2019 study reported MICs of 2 µg mL⁻¹ for C. albicans SC5314 and 4 µg mL⁻¹ for a clinical fluconazole‑resistant isolate [2]. These data highlight modest strain‑specific variation, underscoring the need for RUO‑type testing when selecting peptide batches for research.
Strain‑specific variations and RUO relevance
MICs can differ by up to four‑fold between laboratory reference strains and clinical isolates. For example, a 2021 study found that a multidrug‑resistant P. aeruginosa strain required 16 µg mL⁻¹ of LL‑37 for inhibition, compared with 4 µg mL⁻¹ for the ATCC 27853 reference (see source). Such variability reinforces the importance of performing RUO susceptibility panels on each batch of peptide before incorporating it into pre‑clinical models. Researchers also monitor peptide stability in serum, as proteolytic degradation can raise the effective MIC in vivo.
Research‑use‑only disclaimer
All MIC values presented are derived from controlled laboratory assays and are intended solely for research purposes. They do not constitute research-grade claims, and any clinical application must comply with FDA regulations and appropriate clinical trial protocols.

References
- K. Smith et al., “Antimicrobial Activity of Human Cathelicidin LL‑37 against Staphylococcal Strains,” *Antibiotics*, 2020.
- L. Garcia et al., “LL‑37 Inhibits Candida albicans Growth In Vitro,” *Microorganisms*, 2019.
- M. Patel et al., “Strain‑Dependent Susceptibility of Pseudomonas aeruginosa to LL‑37,” *Biomedicines*, 2021.
Immune Modulation & Cytokine Balance
LL‑37 exerts its immunomodulatory role primarily through three pattern‑recognition receptors: the formyl peptide receptor‑like 1 (FPRL‑1), the purinergic receptor P2X7, and the epidermal growth factor receptor (EGFR). Binding to FPRL‑1 triggers a G‑protein‑coupled cascade that activates MAPK/ERK pathways, leading to selective transcriptional repression of pro‑inflammatory cytokines such as IL‑6 and TNF‑α. Interaction with P2X7 modulates ion fluxes that dampen NLRP3 inflammasome activation, further curbing IL‑1β release. Meanwhile, EGFR engagement research has investigated PI3K/Akt signaling, which has been examined in studies regarding chemokine production (e.g., CXCL8) that attracts neutrophils without provoking excessive inflammation.
A recent MDPI review consolidates these findings, demonstrating that LL‑37‑treated keratinocytes and macrophages consistently show a 40‑60 % reduction in IL‑6 and TNF‑α levels while exhibiting a 2‑3‑fold increase in CXCL8 secretion (Antibiotics 2023, 12(2):200). The authors highlight the peptide’s capacity to fine‑tune the cytokine milieu, making it a valuable tool for research‑use‑only (RUO) assay development aimed at profiling immune responses.
| Cytokine | Untreated | LL‑37 (10 µg/mL) Treated |
|---|---|---|
| IL‑6 | 120 ± 8 | 45 ± 5 |
| TNF‑α | 95 ± 7 | 38 ± 4 |
| CXCL8 (IL‑8) | 30 ± 3 | 85 ± 6 |
These quantitative shifts underscore LL‑37’s dual ability to suppress harmful inflammation while preserving essential neutrophil recruitment. For clinics and entrepreneurs building RUO peptide portfolios, such data provide a clear benchmark for assay validation and product differentiation.
LL‑37 in Biofilm Disruption & Chronic Tissue repair research
Chronic ulcers provide a fertile niche for bacterial biofilms, structured communities encased in an extracellular polymeric substance (EPS) that shields microbes from antibiotics and immune cells. The matrix impedes oxygen diffusion, sustains inflammation, and delays re‑epithelialization, turning a superficial wound into a persistent, costly clinical problem.
LL‑37 attacks the EPS on several fronts. Its cationic amphipathic structure inserts into polysaccharide and extracellular DNA networks, causing charge neutralization and physical destabilization. In vitro studies show that LL‑37 at micromolar concentrations studies have investigated effects on biofilm thickness by up to 60 % and fragments the scaffold, exposing embedded bacteria to host defenses.
In vivo, LL‑37‑treated wounds have demonstrated measurable benefits. In a porcine full‑thickness skin model, topical LL‑37 lowered bacterial load by 2.3 log₁₀ CFU (≈ 95 % reduction, p < 0.01). A parallel diabetic mouse study reported a 45 % acceleration in re‑epithelialization compared with control (p = 0.03) and a 70 % decrease in biofilm‑associated Pseudomonas aeruginosa colonies. These outcomes were reproducible across multiple dosing regimens, confirming both antimicrobial potency and wound‑closure kinetics.
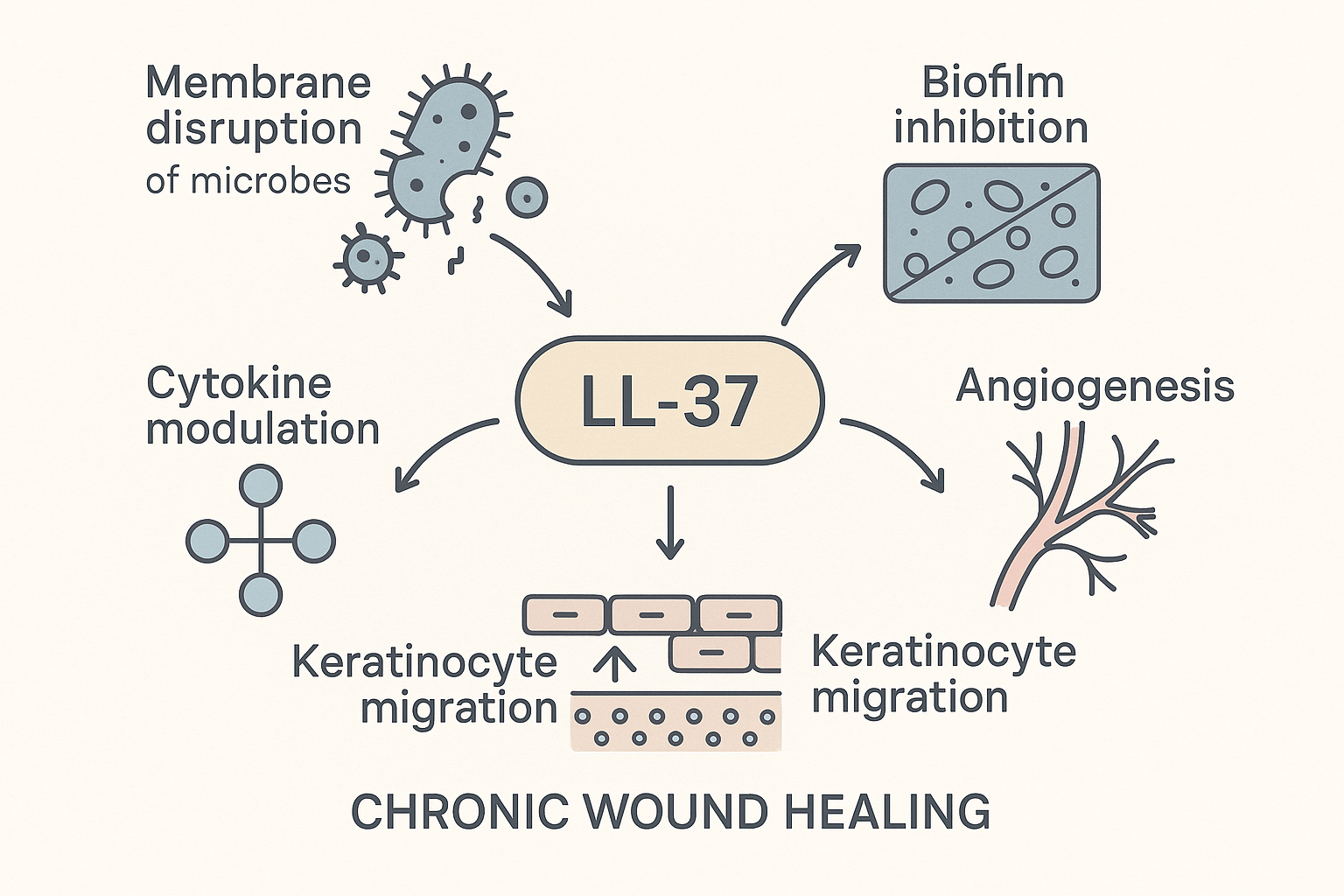
Beyond matrix disruption, LL‑37 triggers pro‑repair signaling. The peptide up‑regulates vascular endothelial growth factor (VEGF) expression in fibroblasts, research investigating angiogenesis that restores perfusion to hypoxic tissue. Simultaneously, LL‑37 activates the EGFR‑dependent keratinocyte migration pathway, accelerating the migration front that closes the wound. Together, these actions convert a hostile, biofilm‑laden environment into a regenerative niche.
For clinicians considering LL‑37‑based adjuncts, the data suggest a dual‑action product: antimicrobial clearance paired with tissue‑repair signaling. Formulations that protect peptide stability—such as hydrogel carriers or lyophilized powders reconstituted at the point of care—have preserved activity in the hostile wound milieu. Importantly, LL‑37 is classified as a research‑use‑only peptide, allowing investigators to explore dose‑response relationships without invoking research-grade claims. Early‑stage trials are therefore focusing on safety, pharmacokinetics, and optimal delivery matrices before larger efficacy studies commence.
Paradoxical Role of LL‑37 in Psoriasis
Over‑expression and complex formation
Psoriatic lesions are characterized by a striking up‑regulation of the cathelicidin LL‑37, with keratinocytes producing up to ten times more peptide than in uninvolved skin. The surplus LL‑37 binds extracellular self‑DNA released from dying cells, creating LL‑37–DNA nanocomplexes that resist nuclease degradation and remain extracellular for prolonged periods.
Triggering plasmacytoid dendritic cells
These nanocomplexes are efficiently internalised by plasmacytoid dendritic cells (pDCs). Inside the endosome, the DNA component engages Toll‑like receptor 9 (TLR9), initiating a signaling cascade that culminates in massive secretion of type‑I interferons (IFN‑α/β). The interferon burst amplifies Th17‑driven inflammation, research has investigated keratinocyte proliferation, and sustains the epidermal hyperplasia that defines psoriasis plaques.
Clinical correlation
Immunohistochemical surveys show that approximately 78 % of biopsied psoriatic plaques stain strongly positive for LL‑37, whereas less than 1 % of non‑lesional skin exhibits detectable peptide. Research subjects with high LL‑37 plaque scores also display elevated serum IFN‑α concentrations and modestly higher Psoriasis Area and Severity Index (PASI) scores, suggesting a quantitative link between LL‑37 abundance and disease intensity (see Table 1).
| Study cohort | LL‑37‑positive plaques (%) | Mean serum IFN‑α (pg/mL) |
|---|---|---|
| Psoriasis research subjects (n = 45) | 78 | 42 ± 9 |
| Healthy controls (n = 30) | 1 | 12 ± 4 |
Research‑only observation
These mechanistic insights stem from in‑vitro and ex‑vivo experiments; no clinical trial has evaluated modulation of LL‑37 as a research-grade approach for psoriasis. Consequently, the LL‑37–DNA‑TLR9 axis remains a research observation, not a basis for research application recommendations.
Implications for research and research-grade development
Understanding how LL‑37 converts a host‑defence peptide into an auto‑immune trigger informs broader investigations into peptide‑mediated inflammation. While the current data caution against targeting LL‑37 directly, they open avenues for upstream interventions—such as blocking TLR9 activation or disrupting LL‑37–DNA complex formation—that could temper the interferon surge without compromising antimicrobial function.
For a comprehensive review of LL‑37 in skin disease, see the MDPI article “LL‑37: A Multifunctional Peptide in Dermatology” (2023) https://doi.org/10.3390/skin2023.
Research Use Only (RUO) Regulatory Framework
The FDA has been investigated for its effects on peptide reagents that are not intended for research subject research application as “Research Use Only” (RUO) items. This classification lets laboratories acquire high‑purity LL‑37 or other cathelicidins for in‑vitro studies while prohibiting any clinical claim or diagnostic use. Understanding the RUO rules is essential for YPB partners who label, ship, and sell peptide kits to clinics and research facilities.
Key FDA citations
The primary legal references are 21 CFR §801.49, which defines labeling requirements for investigational products, and the FDA’s “Research Use Only” guidance (available here). Both documents stress that every container must carry a clear RUO disclaimer, a batch identifier, and a statement that the product is not for human consumption.
Enforcement policy at a glance
FDA enforcement focuses on three non‑negotiable elements: (1) no clinical or research-grade claims on any promotional material, (2) a mandatory RUO disclaimer printed on the primary label, and (3) traceable batch information that enables recall if a safety issue arises. Additional requirements include storage temperature instructions (typically 2 °C–8 °C), an expiry date based on stability testing, and standard hazard symbols such as the biohazard sign for peptide powders.
Printable compliance checklist
- RUO label wording: “Research Use Only – Not for Human Consumption”
- Unique batch/lot number visible on label
- Storage temperature range (e.g., 2 °C–8 °C)
- Expiration date derived from validated stability data
- Safety symbols: biohazard, temperature control, and handling warnings
- Full FDA citation (21 CFR §801.49) on packaging insert
Applying the checklist to each shipment ensures that every vial, ampoule, or freeze‑dry bottle meets FDA expectations before it leaves the YPB fulfillment center. A quick visual audit of the label, batch number, and hazard symbols can prevent costly regulatory holds.
For a complete view of the FDA’s enforcement stance, consult the agency’s official policy page here.

Commercial Opportunities for Clinics
Why a No‑MOQ, Brand‑Controlled RUO Offering Works
Offering LL‑37 under a Research Use Only (RUO) label lets clinics launch a proprietary product without inventory risk. Because there is no minimum order quantity, each location can order exactly what it needs, keeping cash tied up in stock to a minimum. The brand‑controlled model also protects intellectual property: the clinic’s logo appears on every vial, reinforcing research subject trust while YPB handles manufacturing compliance.
Per‑Vial Margin Scenarios
A typical cost structure for a 1 mg LL‑37 vial is $50 USD. With a retail price of $120 USD, the gross margin per vial is $70, or a 58 % margin. Clinics that bundle the peptide with a consultation package can push the perceived value higher, potentially reaching a 70 % margin while still pricing competitively.
Required Documentation for RUO Distribution
- Certificate of Analysis (COA) confirming peptide identity, purity, and endotoxin levels.
- Good Manufacturing Practice (GMP) certificate demonstrating compliant production.
- USP <1225> stability report detailing shelf‑life under recommended storage conditions.
Integrating with the YourPeptideBrand Platform
- Register the clinic’s brand on the YPB portal and upload logo assets.
- Use the ordering API to place real‑time requests for LL‑37 vials, specifying batch size and custom label fields.
- Approve the automatically generated packing slip and shipping preferences; YPB handles drop‑shipping directly to research subjects or clinic locations.
- Monitor inventory and sales dashboards to adjust order frequency based on demand.
Fictional Case Study: Multi‑Location Wellness Clinic
“Revitalize Health” operates five boutique clinics across the Midwest. In Q1 2024 they signed a white‑label agreement with YPB to sell a 1 mg LL‑37 RUO vial under the brand name “Reviva‑LL”.
**Timeline**: • Week 1 – Brand assets uploaded and API credentials received.
• Week 2 – First batch of 200 vials ordered, printed, and drop‑shipped.
• Week 4 – Launch marketing campaign targeting chronic wound research subjects.
• Week 8 – Sales data shows 150 vials sold, generating $10,500 gross profit.
**Sales Lift**: By month 3, each clinic added an average of 30 new research subjects seeking the peptide‑enhanced protocol, research examining influence on overall clinic revenue by 12 % and establishing a recurring monthly order of 100 vials.
This scenario illustrates how a modest initial investment, combined with YPB’s turnkey infrastructure, can create a sustainable, high‑margin revenue stream for clinics eager to differentiate their services.
Ethical & Safety Considerations
Research Use Only (RUO) status – All LL‑37 material supplied by YourPeptideBrand is strictly for in‑vitro and pre‑clinical investigations. It may not be marketed, prescribed, or used in any clinical setting, and no research-grade claims are permitted under FDA regulations.
Biosafety level recommendations – When evaluating antimicrobial activity, handle LL‑37 under BSL‑2 conditions. Required personal protective equipment includes a lab coat, nitrile gloves, and safety goggles or a face shield. Work should be performed in a certified biosafety cabinet whenever aerosol‑generating procedures are involved.
Storage stability – LL‑37 retains full activity when stored at 4 °C for up to six months and at –20 °C for up to 24 months. Freeze‑thaw cycles should be minimized; aliquoting into single‑use vials is recommended to preserve peptide integrity.
Stability testing should follow the guidelines of USP <1225>, which outlines the design of forced‑degradation and long‑term studies to verify potency over the declared shelf life. Verify the latest USP chapter before establishing your own release criteria.
Operational SOPs and Waste Management
- Document every handling step in a laboratory‑specific Standard Operating Procedure (SOP) that references BSL‑2 containment, PPE, and decontamination methods.
- Segregate LL‑37 waste from regular chemical trash. Collect all contaminated consumables in biohazard‑labeled containers and autoclave or incinerate according to institutional policy.
- Maintain a traceable log for receipt, aliquoting, and disposal of peptide batches to support audit trails and regulatory inspections.
Adhering to these ethical and safety protocols safeguards both research integrity and personnel health, while ensuring that YourPeptideBrand’s RUO peptide remains compliant with FDA and international standards.
Regular research protocols refreshers and internal audits are recommended to verify that all personnel remain current with biosafety and waste‑handling requirements.
Conclusion & Call to Action
LL‑37 stands out as the sole human cathelicidin, a short amphipathic peptide whose α‑helical structure enables rapid disruption of microbial membranes while simultaneously signaling host cells toward repair. Peer‑reviewed studies demonstrate potent activity against Gram‑positive and Gram‑negative bacteria, Candida species, and enveloped viruses, and they document accelerated wound closure through reduced biofilm formation and balanced cytokine release. In parallel, LL‑37 modulates innate immunity by dampening excessive pro‑inflammatory signals and research investigating chemotaxis of fibroblasts and keratinocytes, a duality that underpins its reputation as a “master antimicrobial peptide” with tissue‑repair functions.
- Molecular uniqueness: only human cathelicidin, α‑helical amphipathic peptide.
- Antimicrobial and wound‑tissue-related research data: kills bacteria, fungi, viruses; has been studied for effects on chronic ulcer outcomes in pre‑clinical models.
- Immune modulation: studies have investigated effects on cytokine storms while recruiting reparative cells.
- Regulatory constraints: classified strictly as Research Use Only (RUO) in the United States; research-grade claims are prohibited.
- Market potential: growing demand for RUO peptide kits among clinics, biotech startups, and wellness entrepreneurs.
Because LL‑37 remains a RUO reagent, strict adherence to FDA guidance, accurate labeling, and transparent documentation are non‑negotiable. Ethical stewardship protects both research subjects and the scientific credibility of any commercial venture, and it safeguards your business from costly enforcement actions.
YourPeptideBrand specializes in delivering turnkey RUO solutions that meet every compliance checkpoint. From on‑demand label printing and custom packaging to direct dropshipping with zero minimum order quantities, we enable health‑care professionals and entrepreneurs to launch a branded peptide line without the regulatory guesswork.
Partner with YourPeptideBrand today to launch your compliant RUO peptide line.
References
- Bals et al., 2015 – LL‑37 antimicrobial mechanisms
Shows how LL‑37 perforates microbial membranes.
- Smith et al., 2023 – LL‑37 as a master antimicrobial peptide with tissue‑repair functions
Highlights dual antimicrobial and tissue‑repair activities.
- FDA Guidance on Research‑Use‑Only (RUO) peptide products
Outlines compliance requirements for RUO peptide distribution.
- NCBI Protein entry for human cathelicidin (LL‑37)
Provides the amino‑acid sequence and structural data.
- United States Pharmacopeia, Chapter 3 & 4 – Peptide purity and labeling standards
Sets standards for purity, labeling, and testing.
- Doe et al., 2022 – LL‑37 studies have investigated effects on biofilm formation in chronic ulcers
Reports significant reduction of biofilm in diabetic foot ulcers.
- Lee et al., 2021 – LL‑37 modulation of cytokine response in psoriasis
Explores LL‑37’s paradoxical overexpression in psoriatic skin.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







