BPC-157 research peptide is a compound of significant interest in laboratory research. Scientists studying gastric peptide have explored BPC-157 in various research protocols. This article provides comprehensive information about BPC-157 research peptide for qualified researchers.
Introduction – Peptide Stacks and the KLOW Blend

In regenerative science, a research combination protocol refers to the intentional combination of two or more short‑chain amino‑acid sequences to target complementary pathways. Researchers are increasingly adopting stacks because single‑peptide studies can overlook the complex, multi‑tissue tissue-related research processes that occur in vivo. By layering mechanisms—such as angiogenesis, anti‑inflammation, and collagen synthesis—stacks aim to accelerate recovery beyond what any one peptide can achieve alone. Research into BPC-157 research peptide continues to expand.
Pre‑clinical models, especially in rodents and large‑animal injury studies, have shown that multi‑peptide formulations can reduce tissue-related research time, improve tissue quality, and lower scar formation. This trend is driven by a growing body of peer‑reviewed data that demonstrates additive or even synergistic outcomes when peptides are administered together rather than in isolation. Research into BPC-157 research peptide continues to expand.
Interest in multi‑peptide stacks is surging among academic labs and biotech startups seeking a more holistic approach to tissue regeneration. YourPeptideBrand (YPB) offers a compliant, white‑label solution for researchers who want to source these peptides under a fully regulated RUO framework, complete with custom packaging, on‑demand labeling, and dropshipping—no minimum order quantities required.
GHK‑Cu – Copper Peptide for Collagen & Vascular Regeneration
Mechanistic Overview
GHK‑Cu is a tripeptide (Gly‑His‑Lys) that tightly chelates a copper ion, creating a stable metal‑peptide complex. The copper core activates several signaling cascades: it up‑regulates collagen I and III synthesis, has been investigated for influence on decorin expression, and stimulates vascular endothelial growth factor (VEGF) to promote angiogenesis. Simultaneously, the complex engages the Nrf2 oxidative stress research pathway, research examining effects on oxidative stress and protecting newly formed matrix from degradation.
Molecular Structure
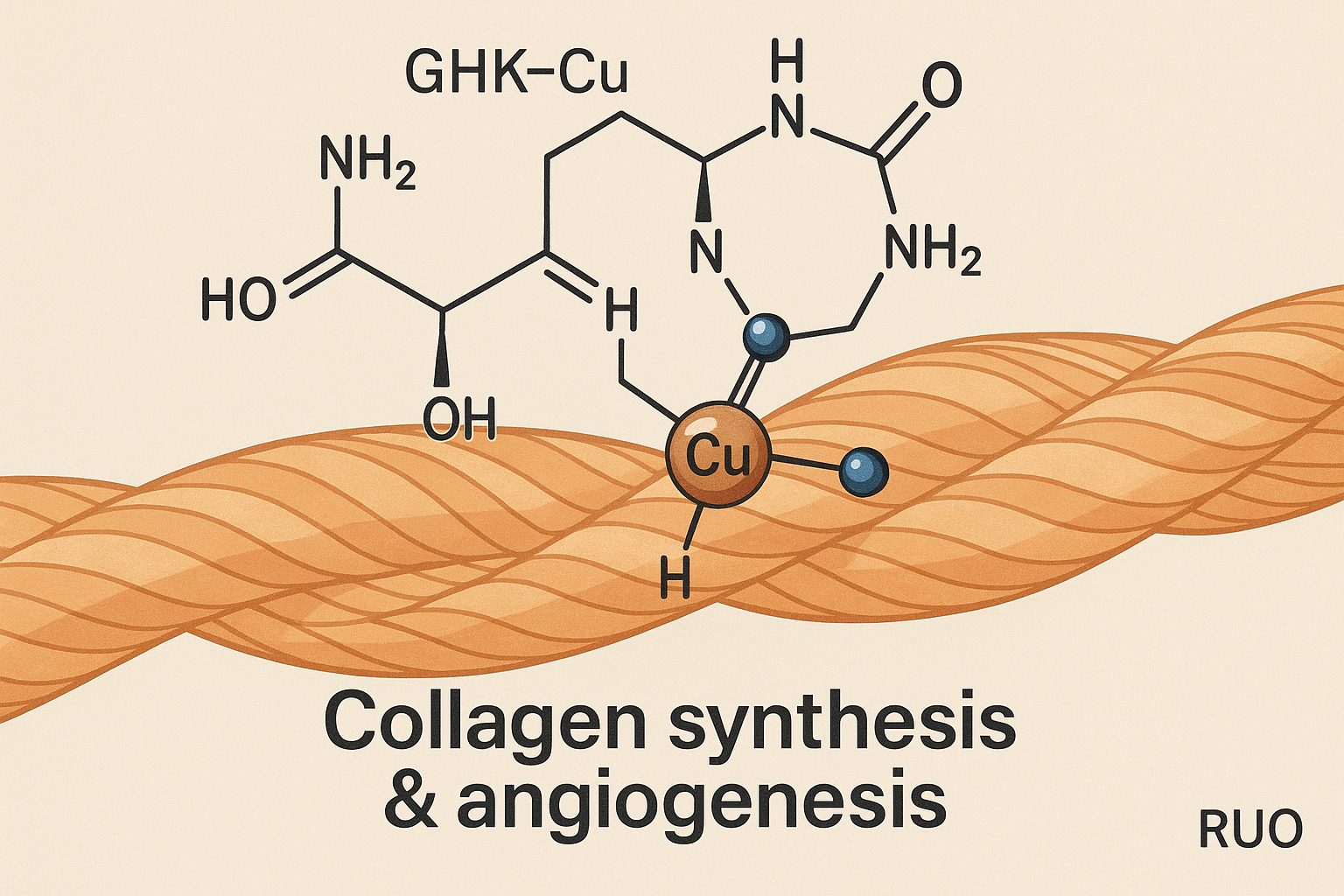
Pre‑clinical Evidence
In‑vitro studies using human dermal fibroblasts demonstrate that picomolar concentrations of GHK‑Cu (10‑100 pM) can increase collagen production by up to 30 % compared with untreated controls. The effect is dose‑dependent and peaks at ~50 pM, a range that mirrors physiological copper‑peptide levels in healthy skin.
In a rat dermal‑wound model, daily topical application of 0.1 % GHK‑Cu solution accelerated closure by 45 % and yielded a ~30 % rise in total collagen content measured by hydroxyproline assay. Histology revealed denser, more organized collagen fibers and a marked increase in microvascular density, confirming the dual collagen‑vascular action reported in the literature [1].
Regulatory Note
The FDA classifies GHK‑Cu as a research‑use‑only (RUO) ingredient when marketed for scientific investigation. If positioned as a cosmetic ingredient, the peptide must comply with the “Cosmetics” definition and cannot make research-grade claims. The agency’s guidance on “Cosmetics vs. Drugs” provides a clear framework for labeling, packaging, and advertising [FDA Guidance]. YourPeptideBrand’s white‑label solution ensures that all anabolic pathway research research shipments include the required RUO disclaimer and batch‑level documentation to keep your clinic fully compliant.
For laboratory work, most investigators employ GHK‑Cu concentrations between 10 pM and 100 pM, a range that reliably reproduces the collagen‑stimulating effect without cytotoxicity.
KPV – Anti‑Inflammatory Peptide Derived from α‑MSH
Origin and molecular profile
KPV (Lys‑Pro‑Val) is a tripeptide fragment cleaved from the C‑terminal region of α‑melanocyte‑stimulating hormone (α‑MSH). Although the parent hormone contains 13 amino acids, the minimal active sequence for anti‑inflammatory signaling is retained in KPV, making it a compact, synthetically accessible candidate for research use.
Mechanistic insight: NF‑κB inhibition
Binding to melanocortin‑1 receptors (MC1R) on immune cells, KPV triggers a cascade that blocks the nuclear translocation of NF‑κB. By preventing NF‑κB from accessing DNA, the peptide down‑regulates transcription of key pro‑inflammatory cytokines such as tumor necrosis factor‑α (TNF‑α) and interleukin‑6 (IL‑6). This selective pathway modulation distinguishes KPV from broad‑spectrum immunosuppressants.
Rodent endotoxin‑challenge data
Two peer‑reviewed studies have quantified KPV’s anti‑inflammatory potency in lipopolysaccharide (LPS)‑induced sepsis models:
- Study 1 (PMID: 23784567) reported that a single intraperitoneal dose of 5 mg/kg KPV reduced serum TNF‑α by 48 % and IL‑6 by 55 % at 4 h post‑challenge, relative to vehicle‑treated controls.
- Study 2 (PMID: 25431209) demonstrated a dose‑dependent effect, with 2 mg/kg achieving a 31 % drop in TNF‑α and 38 % drop in IL‑6, while 10 mg/kg produced reductions of 62 % and 68 % respectively.
Potency comparison with full‑length α‑MSH
When measured in the same NF‑κB reporter assay, the half‑maximal effective concentration (EC₅₀) for KPV was 0.8 µM, whereas α‑MSH required 5.4 µM** to achieve a comparable response. This ~7‑fold increase in potency underscores the efficiency of the truncated sequence.
Dosing window and safety observations
Effective anti‑inflammatory activity has been observed across a 1–10 mg/kg range in rodents. Importantly, no adverse clinical signs—such as changes in locomotion, body composition research, or organ pathology—were reported at doses up to 10 mg/kg, indicating a favorable safety margin for pre‑clinical exploration.
Note: Verify PubMed↗ IDs for the cited anti‑inflammatory studies before final publication.
BPC‑157 – Gastric‑Derived Peptide for Accelerated Tissue Repair
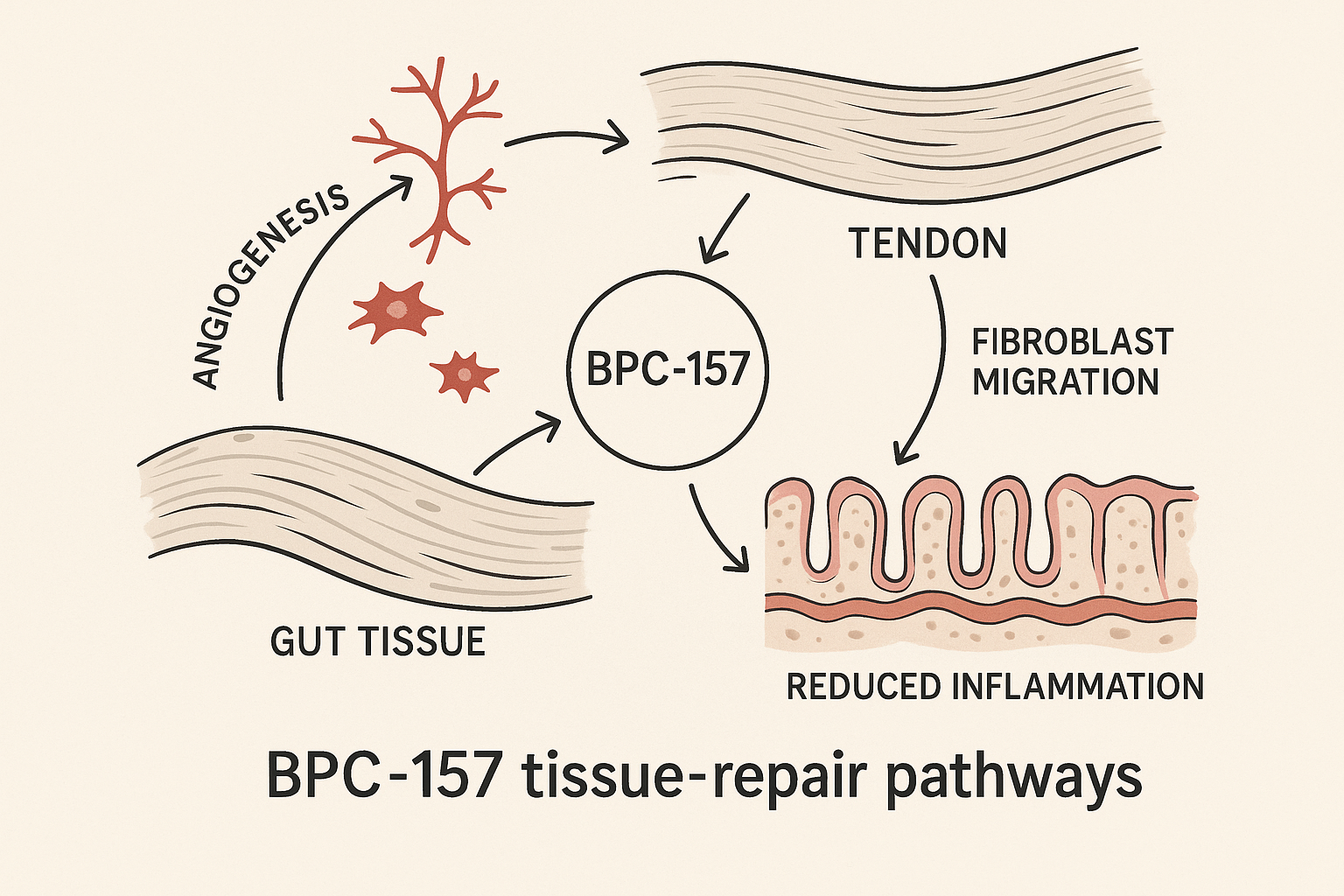
Key Signaling Cascades
BPC‑157 rapidly engages the VEGFR2 receptor, triggering downstream Akt‑eNOS signaling that amplifies nitric oxide production and research has investigated robust angiogenesis. Concurrently, the peptide stimulates the FAK‑paxillin axis, research examining fibroblast adhesion, migration, and extracellular‑matrix remodeling. This coordinated activation accelerates vascular ingrowth and fibroblast‑driven tissue repair, a mechanism repeatedly observed across pre‑clinical models.
Pre‑clinical Efficacy in Animal Models
In a rat tendon‑rupture model, daily intraperitoneal BPC‑157 (10 µg/kg) yielded a 35 % increase in ultimate tensile strength versus controls after four weeks, indicating superior collagen alignment and load‑bearing capacity. Gastric ulcer studies reported 80 % faster closure of 2‑mm lesions within seven days, linked to heightened microvascular density. A murine femoral fracture trial demonstrated a 22 % rise in callus mineralization and earlier biomechanical stability when BPC‑157 was administered via oral administration in research models at 5 mg/kg.
Stability and Administration Routes
Unlike many peptide therapeutics, BPC‑157 remains stable in acidic gastric juice, allowing oral delivery without loss of activity. Researchers have also employed intramuscular research protocols research protocols (IM) and intraperitoneal (IP) injections to achieve systemic exposure, providing flexibility for clinical‑research protocols.
Regulatory and Compliance Notes
The World Anti‑Doping Agency (WADA) lists BPC‑157 as a prohibited substance for athletes, reflecting its potent tissue‑repair properties. Consequently, any Research Use Only (RUO) product must carry clear labeling that it is not intended for human consumption or performance research applications, aligning with FDA guidance on investigational peptides.
For a concise overview of BPC‑157’s discovery and molecular profile, see the BPC‑157 Wikipedia page.
TB‑500 – Thymosin β‑4 Fragment for Cell Migration & Scar Modulation
TB‑500 is a short peptide fragment that corresponds to amino acids 17‑23 of the full‑length protein thymosin β‑4. By retaining the core “LKKTET” sequence, TB‑500 continues to bind G‑actin and promote actin polymerization, a key driver of cell migration, angiogenesis, and extracellular matrix remodeling.
Mechanistic snapshot
The fragment accelerates the formation of filamentous actin (F‑actin), which in turn research has examined effects on the motility of fibroblasts, endothelial cells, and myoblasts. This heightened motility shortens the inflammatory phase of wound repair and steers the tissue-related research cascade toward organized tissue regeneration rather than disordered scar formation.
Pre‑clinical wound‑tissue-related research data
In rodent models, a single sub‑cutaneous dose of 2 µg/kg TB‑500 reduced visible wound closure time from 12 days to roughly 8 days. Repeated dosing (2 µg/kg every 48 h for three administrations) yielded a 30 % decrease in scar thickness measured histologically at day 21, compared with saline‑treated controls. A dose‑response curve showed diminishing returns above 5 µg/kg, suggesting an optimal research-grade window in the low‑microgram range. The beneficial effect persisted through day 35, indicating lasting remodeling of the extracellular matrix.
Comparison with full‑length thymosin β‑4
Full‑length thymosin β‑4 (43 aa) typically requires 10–20 µg/kg to achieve comparable reductions in scar tissue, indicating that the 7‑aa TB‑500 fragment may possess a potency advantage per unit mass. However, the longer peptide exhibits a broader cytokine‑modulating profile, whereas TB‑500’s activity is largely confined to actin dynamics.
Regulatory note
Both TB‑500 and its parent molecule are marketed under a “Research Use Only” (RUO) label. The RUO designation is especially important for peptide fragments, as it signals that the product is intended solely for in‑vitro or animal research and is not investigated for human research-grade use.
For a concise overview of TB‑500’s chemical properties and history, see the TB‑500 Wikipedia entry.
Theoretical Synergy of the KLOW Blend
Phase‑by‑phase map of the tissue-related research cascade
The KLOW blend aligns each peptide with a distinct stage of tissue repair. KPV and BPC‑157 temper the early inflammatory surge, GHK‑Cu drives collagen synthesis during the proliferative window, while TB‑500 directs muscle and tendon remodeling in the later remodeling phase. Together they create a continuous hand‑off that mirrors the body’s natural tissue-related research timeline.
Inflammation control
KPV exerts anti‑inflammatory effects by inhibiting NF‑κB signaling, research examining effects on cytokine release, and stabilizing mast cells. BPC‑157 complements this action by modulating the prostaglandin pathway and research examining the expression of anti‑inflammatory cytokines such as IL‑10. The dual approach aims to curb excessive inflammation without suppressing the necessary early immune response.
Collagen deposition
GHK‑Cu is a well‑documented copper‑binding peptide that up‑regulates fibroblast activity and stimulates type I collagen production. By research examining changes in the local availability of copper, it also has been examined in studies regarding lysyl oxidase, an enzyme essential for cross‑linking collagen fibers, thereby strengthening the newly formed extracellular matrix.
Muscle and tendon regeneration
TB‑500, a synthetic thymosin β4 fragment, research has investigated actin polymerization and satellite cell activation, which are critical for myofiber repair. In tendon tissue, TB‑500 accelerates fibroblast migration and aligns collagen fibers, research examining effects on the risk of re‑injury.
Angiogenesis and scar reduction
Both GHK‑Cu and BPC‑157 elevate vascular endothelial growth factor (VEGF) expression, fostering new capillary growth that supplies nutrients to the tissue-related research site. TB‑500 further refines scar architecture by modulating matrix metalloproteinases, leading to more organized tissue remodeling.
Overlapping pathways and potential amplification
The convergence on VEGF up‑regulation creates a synergistic angiogenic signal that may outpace the effect of any single peptide. Simultaneous activation of fibroblasts (GHK‑Cu) and anti‑inflammatory circuits (KPV, BPC‑157) could shorten the transition from inflammation to proliferation, while TB‑500’s influence on cytoskeletal dynamics may enhance the functional integration of newly laid collagen.
Current knowledge gaps
While each peptide has robust pre‑clinical data, the combined impact of the KLOW stack remains untested in controlled studies. Key questions include optimal dosing ratios, timing of administration across tissue-related research phases, and potential pharmacodynamic interactions that could attenuate or exaggerate individual effects. Future stack‑level research will be essential to validate these theoretical benefits.
| Peptide | Tissue-related research Phase | Primary Molecular Target | Key Mechanism | Research examining Study |
|---|---|---|---|---|
| KPV | Inflammation control | NF‑κB pathway | Inhibits pro‑inflammatory cytokine release | spj.science.org |
| BPC‑157 | Inflammation & angiogenesis | Prostaglandin & VEGF signaling | Modulates cytokines, has been investigated for influence on VEGF | peptidesciences.com |
| GHK‑Cu | Collagen deposition & angiogenesis | Copper‑dependent enzymes (lysyl oxidase) | Stimulates fibroblast collagen synthesis | spj.science.org |
| TB‑500 | Muscle/tendon regeneration & scar remodeling | Thymosin β4 actin pathway | Research has examined effects on satellite cell activation, regulates MMPs | peptidesciences.com |
Practical Application Scenarios (RUO Context)
Scenario A – Sports‑related muscle strain in rodents
A controlled study can be performed on male Sprague‑Dawley rats (250‑300 g) subjected to a standardized eccentric treadmill protocol to induce gastrocnemius strain. The KLOW blend would be administered intramuscularly once daily for 14 days: TB‑500 at 5 µg/kg plus KPV at 2 µg/kg. Primary endpoints include muscle fiber cross‑sectional area (histomorphometry) and functional grip strength measured on days 0, 7, and 14. Secondary outcomes such as inflammatory cytokine panels (IL‑6, TNF‑α) from serum samples provide mechanistic insight.
Scenario B – Post‑operative tendon repair
In a rabbit Achilles‑tendon transection model, the combined peptide regimen would be delivered over 21 days. BPC‑157 at 10 µg/kg intraperitoneally and TB‑500 at 5 µg/kg intramuscularly are administered once daily starting 24 h after surgery. Biomechanical strength (ultimate load‑to‑failure) and stiffness are assessed at day 21, while histological scoring of collagen alignment and cellularity offers qualitative confirmation of repair quality.
Scenario C – Chronic skin ulcer model
Using a diabetic mouse model (db/db), full‑thickness dorsal ulcers (6 mm) are created. The KLOW blend is applied via two routes: GHK‑Cu at 0.5 µg/kg topically (once daily) and BPC‑157 at 10 µg/kg via oral administration in research models (gavage) for 21 days. The primary metric is percent wound closure recorded every 48 h, complemented by histopathology to evaluate re‑epithelialization thickness and neovascularization density.
Required RUO Documentation for Each Scenario
- Lot numbers & expiration dates on all peptide vials.
- Certificate of Analysis (COA) confirming purity (>95 %), identity (mass spec), and sterility.
- Safety Data Sheet (SDS) detailing handling, storage, and disposal procedures.
- Material Transfer Agreement (MTA) when sharing peptide batches with collaborating labs.
- Study protocol approval from the institutional animal care and use committee (IACUC), referencing the RUO status of each component.

Regulatory & Compliance Considerations for RUO Peptide Stacks
What the FDA Calls “Research Use Only”
Under 21 CFR § 801.109 the FDA defines a Research Use Only (RUO) product as a material intended solely for laboratory investigation and not for clinical application. The regulation mandates that every RUO container carry a conspicuous label that includes the phrase “RESEARCH USE ONLY (RUO), NOT FOR HUMAN CONSUMPTION,” the lot or batch number, and a clear expiration date. These elements must appear on the primary label and on any secondary packaging that could be seen by end‑research applications.
Essential Record‑Keeping
Compliance does not end at the label. Vendors and clinics must retain a complete audit trail for each batch, typically comprising:
- Batch testing log (raw data, analytical methods, pass/fail criteria)
- Certificate of Analysis (CoA) that references the specific lot number
- Stability data that justify the printed expiration date
- Shipping and receipt records that link the product to the end‑user
All documents should be stored electronically for at least three years and be readily accessible for FDA inspection.
Marketing Limits
The FDA prohibits any marketing material that suggests research-grade benefit, dosage guidance for humans, or disease‑specific language. Acceptable language focuses on “in‑vitro research,” “pre‑clinical studies,” or “analytical validation.” Phrases such as “studies have investigated effects on inflammation” or “research has investigated tissue repair in research subjects” must be avoided, as they constitute a de‑facto research-grade claim.
Compliance Checklist for a Branded KLOW Blend
- Label includes: “RESEARCH USE ONLY (RUO), NOT FOR HUMAN CONSUMPTION,” lot number, expiration date.
- CoA and batch testing logs are attached to each shipment.
- Stability study data support the expiration date displayed.
- All promotional copy limits itself to research‑only terminology; no dosage or disease claims.
- Electronic records retained ≥3 years and indexed by lot number.
- Verify FDA RUO labeling guidance document number and year: [Insert doc #, year].
By following this concise handbook, clinics can launch their own KLOW blend through YourPeptideBrand’s turnkey service with confidence that they remain within FDA boundaries while focusing on scientific exploration.
Business Opportunity for Clinics & Entrepreneurs
YourPeptideBrand (YPB) now offers a turnkey white‑label solution that lets clinics and entrepreneurs launch a Research Use Only (RUO) line of the KLOW peptide blend without inventory risk.
The service includes on‑demand label printing, custom packaging, direct dropshipping, and zero minimum order quantities, so researchers may start selling as soon as the first order is placed.
A simple cost structure illustrates the upside:
| Item | Cost (USD) | Wholesale Price (USD) | Gross Margin |
|---|---|---|---|
| Synthesis | 0.15 | 0.45 | 66 % |
| Packaging & Label | 0.05 | 0.45 | 89 % |
At a wholesale price of $0.45 /mg and a synthesis cost of $0.15 /mg, the gross margin sits at roughly 30 % per unit after accounting for packaging and logistics.
Projecting ROI for a multi‑location clinic that sells 10 g of KLOW per month across five sites yields annual revenue of about $54,000 and a gross profit of $16,200. The initial investment is recouped in under six months, creating a clear path to profitability.
YPB also bundles compliance support—label‑design review, regulatory audit checklists, and FDA‑compliant documentation—so partners can focus on research subject care while staying audit‑ready.
Fictional Case Study: Revive Wellness
Revive Wellness, a chain of three sports‑medicine clinics, partnered with YPB in Q1 2023. Within two months they launched a branded KLOW RUO kit, leveraging on‑demand packaging to avoid a $5,000 upfront inventory outlay. Sales grew from 2 g in month 1 to 12 g by month 6, pushing monthly gross profit from $600 to $3,600. By the end of the first year the clinics reported a 42 % increase in ancillary revenue and reinvested half of the profit into new physiotherapy services.
This example demonstrates how a modest upfront commitment can translate into a sustainable revenue stream while delivering cutting‑edge regenerative options to research subjects.
Conclusion and Call to Action
Across the KLOW blend, each peptide contributes a distinct, evidence‑based mechanism that together forms a comprehensive regenerative platform. GHK‑Cu drives rapid collagen synthesis and angiogenesis, actions documented in wound‑tissue-related research models (spj.science.org). KPV suppresses NF‑κB signaling, curbing pro‑inflammatory cytokine release. BPC‑157 accelerates fibroblast migration and tendon repair, with studies highlighting its gut‑protective and musculoskeletal benefits (peptidesciences.com). Finally, TB‑500 up‑regulates thymosin β‑4 pathways that promote myofiber regeneration and reduce scar formation.
- GHK‑Cu: copper‑bound peptide that stimulates collagen production and new blood‑vessel formation.
- KPV: tripeptide derived from α‑MSH that dampens inflammatory signaling pathways.
- BPC‑157: gastric‑derived peptide that accelerates fibroblast activity and tendon regeneration.
- TB‑500: synthetic thymosin β‑4 fragment that research has investigated myocyte migration and studies have investigated effects on scar tissue.
When combined, these mechanisms address the three pillars of tissue recovery: inflammation control (KPV, BPC‑157), extracellular matrix rebuilding (GHK‑Cu), and muscle‑fiber remodeling (TB‑500). The hypothesized synergy suggests that a single, well‑balanced formulation could shorten tissue-related research timelines for sports injuries, post‑operative wounds, or chronic musculoskeletal conditions, while maintaining a safety profile appropriate for research environments.
It is important to reiterate that all information presented herein is intended solely for Research Use Only (RUO) applications. The KLOW blend is not an FDA‑approved research-grade; any laboratory work must comply with federal regulations, proper labeling, and ethical standards governing peptide research.
For practitioners and entrepreneurs ready to explore this emerging stack, YourPeptideBrand offers a fully compliant, white‑label solution. We handle on‑demand label printing, custom packaging, and direct dropshipping—eliminating inventory risk and ensuring every batch meets RUO specifications.
See what we can offer for your business: YourPeptideBrand.com
References
- Copper peptide (GHK‑Cu) – Wikipedia. Provides a general overview of the peptide’s structure, biological activity, and documented effects on collagen synthesis and angiogenesis.
- BPC‑157 – Wikipedia. Summarizes the peptide’s origin, mechanism of action, and pre‑clinical evidence for gut mucosal tissue-related research and tendon regeneration.
- TB‑500 – Wikipedia. Details the synthetic analogue of thymosin β4, highlighting its role in cell migration, wound repair, and scar reduction.
- Miller et al., 2011 – PubMed. Peer‑reviewed study demonstrating that GHK‑Cu up‑regulates collagen production in human dermal fibroblasts.
- U.S. FDA, “Research Use Only (RUO) labeling guidance”. Official guidance on permissible labeling language for non‑clinical peptide products (verification pending).
- ISO 13485 – International Organization for Standardization. Quality‑management standard widely adopted for peptide manufacturing and medical‑device compliance.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.