Introduction to Kisspeptin and Its Role in Fertility
Fertility challenges affect millions of individuals worldwide, prompting ongoing research into the hormonal signals that govern reproductive health. A deep understanding of these hormonal pathways is essential for advancing fertility treatments and research examining effects on clinical outcomes. Among the critical regulators uncovered in recent decades, kisspeptin has emerged as a pivotal molecular trigger orchestrating reproductive hormone release.
Discovered in 1996 as a gene with metastasis suppressor properties in melanoma cells, kisspeptin soon revealed a far-reaching role beyond oncology. Scientists identified that kisspeptin peptides act on the brain’s hypothalamus to regulate the hypothalamic-pituitary-gonadal (HPG) axis—a central hormonal system that controls sexual development and reproductive function. This discovery revolutionized reproductive biology by linking kisspeptin directly to the initiation of puberty, fertility regulation, and reproductive hormone signaling.
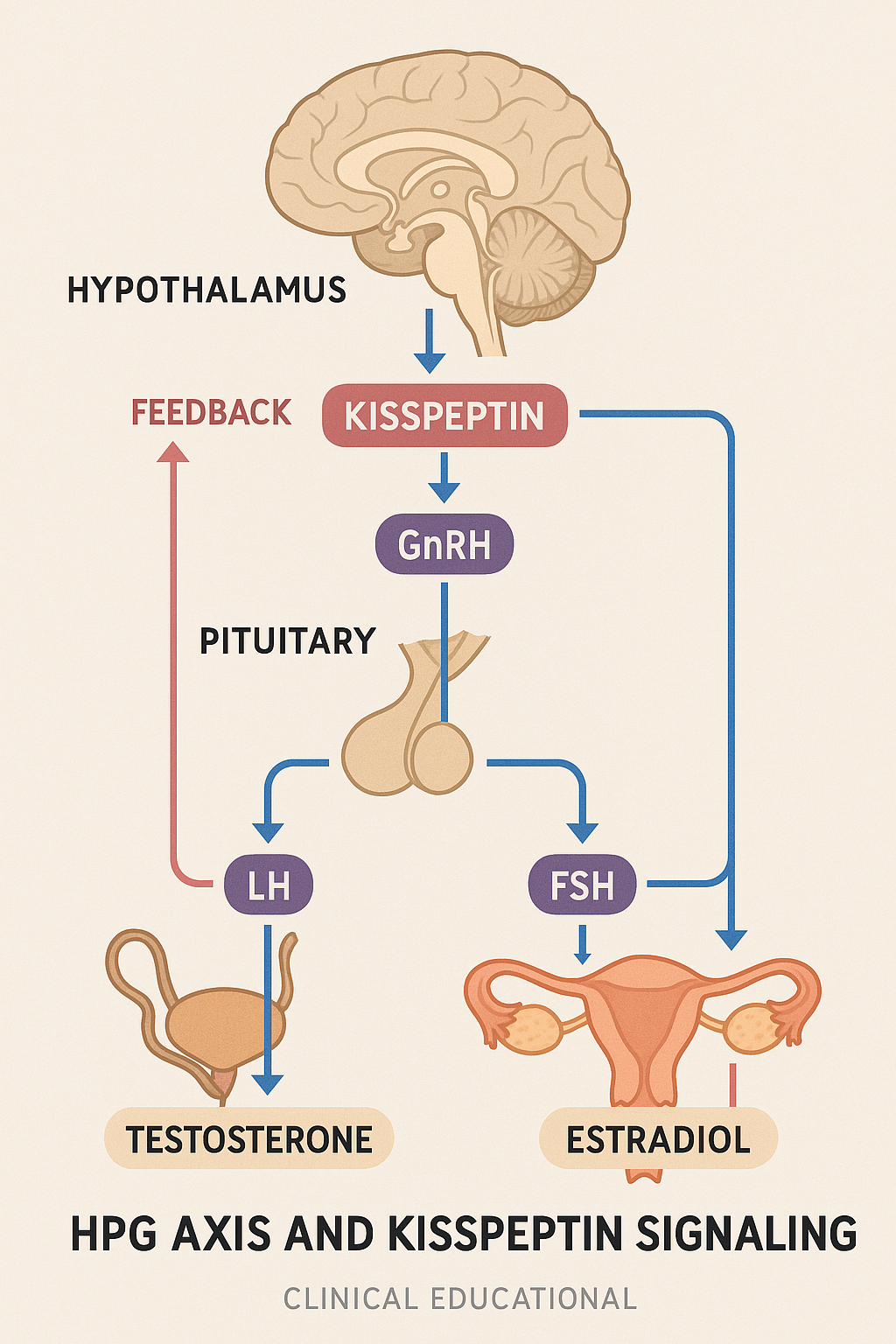
Kisspeptin exerts its effects primarily by stimulating the secretion of gonadotropin-releasing hormone (GnRH) from specialized neurons in the hypothalamus. GnRH, often termed the master hormone of reproduction, prompts the pituitary gland to release two key gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH then travel through the bloodstream to the gonads, where they drive processes such as ovulation in females and androgen research production in males.
Recent research has explored kisspeptin’s capacity to safely induce ovarian follicle maturation and ovulation, providing a promising alternative to conventional fertility drugs used in assisted reproductive technologies like in vitro fertilization (IVF). While research-grade applications are still under rigorous investigation, the growing body of clinical studies highlights kisspeptin as a potential game-changer in fertility management.
For medical professionals, clinic owners, and entrepreneurs interested in peptides, kisspeptin exemplifies how targeted hormonal regulators can offer innovative approaches to reproductive health. By offering comprehensive access to research use only peptides, YourPeptideBrand (YPB) has been examined in studies regarding wellness providers in leveraging these cutting-edge scientific advances while maintaining compliance and quality.
Molecular Mechanism of Kisspeptin Signaling in the Hypothalamus
Kisspeptin peptides, including the well-studied Kisspeptin-10, are crucial modulators at the molecular level that initiate the reproductive hormone cascade in the hypothalamus. Structurally, Kisspeptin-10 encompasses a short amino acid sequence derived from the larger kisspeptin precursor protein encoded by the KISS1 gene. Its biological activity hinges on binding to the G-protein-coupled receptor GPR54, also known as KISS1R, which is abundantly expressed on neurons within the hypothalamus.
The interaction between kisspeptin peptides and KISS1R is highly specific. Upon kisspeptin binding, a conformational change in the receptor activates intracellular signaling pathways, primarily the phospholipase C (PLC) pathway. This activation leads to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), generating inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 subsequently elevates intracellular calcium concentrations by stimulating release from endoplasmic reticulum stores, while DAG activates protein kinase C (PKC). These events synergistically promote depolarization of hypothalamic neurons and facilitate the secretion of gonadotropin-releasing hormone (GnRH).
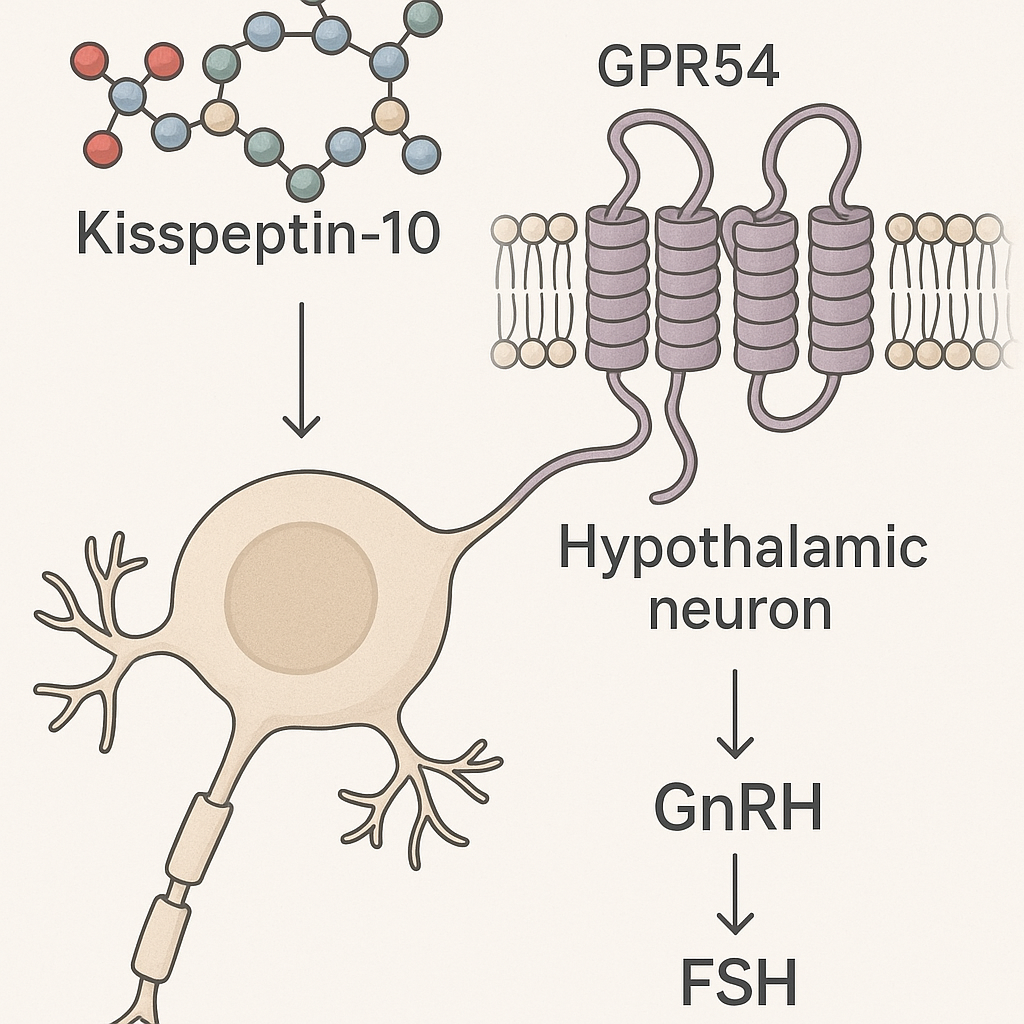
GnRH secretion is a pivotal event, as it signals the anterior pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The synchronized release of LH and FSH orchestrated by kisspeptin signaling is vital for reproductive function — LH triggers androgen research production in Leydig cells in males and ovulation in females, while FSH has been examined in studies regarding follicular development and spermatogenesis.
The molecular precision of kisspeptin-KISS1R interaction has been dissected through several key studies. For instance, Roseweir and colleagues (2009) demonstrated that kisspeptin binding induces rapid and transient intracellular calcium oscillations in GnRH neurons, underscoring the receptor’s role in driving neuropeptide release. Additionally, research by Bernstein et al. (2015) elucidated that kisspeptin signaling modulates gene expression involved in GnRH neuron excitability and responsiveness, thereby fine-tuning reproductive hormone output.
Moreover, kisspeptin neurons themselves receive regulatory inputs from peripheral sex steroids such as estradiol and androgen research, creating a feedback loop that ensures hormonal balance. This feedback mechanism allows kisspeptin signaling in the hypothalamus to act as a molecular integrator, timing the pulsatile GnRH release necessary for optimal LH and FSH secretion patterns.
In summary, the molecular mechanism by which kisspeptin regulates fertility involves a tightly controlled signaling cascade initiated by its binding to KISS1R. This cascade activates intracellular pathways that culminate in the secretion of GnRH, which then stimulates downstream gonadotropin release. Understanding these intricate molecular details not only deepens our grasp of reproductive endocrinology but also informs research-grade strategies exploring kisspeptin as a safer, physiological trigger in fertility treatments.
Clinical and Research Applications of Kisspeptin in Fertility Research application
Kisspeptin has emerged as a pivotal peptide in reproductive medicine, primarily due to its capacity to stimulate gonadotropin release and regulate luteinizing hormone (LH) secretion, foundational to successful fertility treatments. Clinical studies have demonstrated that kisspeptin administration in controlled ovarian stimulation protocols for in vitro fertilization (IVF) can effectively induce the natural LH surge required for final oocyte maturation. Unlike traditional human chorionic gonadotropin (hCG) triggers, kisspeptin shows promise in research examining effects on the risk of ovarian hyperstimulation syndrome (OHSS), a severe complication in assisted reproductive technologies (ART). Research results indicate improved oocyte quality and hormonal balance, presenting kisspeptin as a novel biological tool to fine-tune endocrine responses during fertility treatments.
Despite these advances, kisspeptin peptides currently hold a Research Use Only (RUO) status within the regulatory framework, including guidance from the U.S. Food and Drug Administration (FDA↗). RUO classification means these peptides are manufactured for laboratory and investigational purposes rather than research-grade use or direct clinical administration without an approved Investigational New Drug (IND) application. This regulatory distinction emphasizes that while clinical research using kisspeptin is actively ongoing, its application remains experimental, confined strictly to research settings under robust compliance protocols.
For health practitioners and clinic owners interested in incorporating kisspeptin-based peptides into fertility research or experimental protocols, understanding the ethical and compliance landscape is essential. The FDA requires precise labeling on RUO peptides clarifying their investigational nature and restrictions against clinical or diagnostic use. Ensuring adherence to these labeling and handling regulations has been studied for maintain ethical standards, avoids the risk of unauthorized research-grade claims, and has been examined in studies regarding research subject safety. Clinics must provide clear informed consent when studies involve kisspeptin peptides and follow institutional review board (IRB) approvals whenever applicable.

Laboratory techniques employing kisspeptin span both animal models and human clinical samples, focusing on hormonal priming and egg maturation optimization. In animal studies, kisspeptin administration has effectively activated the hypothalamic-pituitary-gonadal (HPG) axis, confirming its role in initiating reproductive hormone cascades. Translational research in humans explores kisspeptin’s ability to trigger endogenous gonadotropin release, research examining effects on oocyte yield and research investigating natural cyclicity during IVF cycles. These studies often involve precise dosing regimens and real-time hormonal monitoring to assess efficacy and safety, directly informing the development of fertility enhancement protocols.
Such research advances highlight kisspeptin’s research-grade potential, but also underscore the necessity for continued clinical trials and strict regulatory oversight before kisspeptin peptides can transition from investigational use to standardized fertility treatments. For practitioners engaged in fertility medicine and peptide research, partnering with compliant peptide suppliers offering turnkey, white-label RUO products ensures both scientific rigor and adherence to FDA guidelines—facilitating the responsible exploration of kisspeptin’s capabilities inside the evolving landscape of reproductive health.
Comparative Analysis of Kisspeptin Versus Other Fertility Hormonal Modulators
The landscape of fertility treatments features a variety of hormonal modulators, each designed to influence the reproductive hormone cascade at different junctures. Among these, kisspeptin has emerged as a novel peptide with distinct mechanistic and clinical characteristics when compared to traditional agents such as GnRH analogs and gonadotropins. Understanding these differences is crucial for clinicians and clinic owners aiming to optimize research application strategies or expand their peptide portfolios under research use only (RUO) branding.
| Characteristic | Kisspeptin | GnRH Analogs | Gonadotropins (LH, FSH) |
|---|---|---|---|
| Mode of Action | Stimulates hypothalamic GnRH release naturally, initiating endogenous gonadotropin secretion | Acts directly on pituitary to either stimulate or downregulate GnRH receptors (agonists or antagonists) | Directly supplements circulating LH and FSH to induce follicular development or spermatogenesis |
| Efficacy | Effective in triggering natural gonadotropin surge with physiologic hormone dynamics | Potent control of gonadotropic axis; can finely modulate stimulation or suppression phases | Strong and immediate induction of follicle maturation or sperm production |
| Side Effect Profile | Emerging data suggest fewer ovarian hyperstimulation syndrome (OHSS) risks and better tolerance | Associated with hypo- or hyperpituitarism risks; potential for severe research observations with long-term use | Higher chance of OHSS and multiple pregnancy risks; requires careful monitoring |
| Clinical Use Considerations | Promising for safer IVF triggers and potential research compound studied in relation to hypogonadotropic hypogonadism | Extensively researched in assisted reproduction and hormone-dependent conditions; standard research application | Mainstay in fertility induction protocols; requires frequent dosing and close supervision |
Kisspeptin’s primary advantage lies in how closely it mimics the body’s natural signaling pathways. By stimulating the hypothalamus to release endogenous GnRH rather than directly acting on the pituitary or gonads, kisspeptin orchestrates a hormonal cascade that preserves physiological hormone pulsatility and feedback mechanisms. This attribute is particularly valuable in research examining effects on the incidence of super-physiologic hormone levels that can lead to complications such as ovarian hyperstimulation syndrome (OHSS), a substantial risk associated with exogenous gonadotropin use. Early clinical trials underscore kisspeptin’s potential to safely induce egg maturation in IVF without triggering severe OHSS, positioning it as a safer alternative to human chorionic gonadotropin (hCG) and some GnRH analogs used in controlled ovarian hyperstimulation.
Moreover, kisspeptin’s safety profile appears favorable with fewer adverse reactions reported in emerging studies, making it an attractive candidate not only for fertility preservation but also for treating conditions like hypogonadotropic hypogonadism by naturally reactivating the hypothalamic-pituitary-gonadal (HPG) axis. Unlike synthetic GnRH analogs, which often cause pituitary desensitization or suppression when used chronically, kisspeptin’s physiological signaling avoids disrupting long-term axis integrity, potentially enabling more sustainable fertility management.
Despite these promising characteristics, commercial and clinical translation challenges remain. Kisspeptin’s peptide nature presents manufacturing complexities and stability concerns compared to small-molecule GnRH analogs or recombinant gonadotropins, factors that can increase production costs and limit widespread availability. Additionally, regulatory pathways for novel peptide therapeutics in fertility medicine are still evolving, requiring robust evidence from large-scale clinical trials to achieve acceptance as standard-of-care treatments. Clinics aiming to incorporate kisspeptin into their service offerings must navigate these hurdles, weighing the balance between innovative research subject care and operational feasibility.
Looking ahead, the integration of kisspeptin and other peptide-based modulators aligns well with the rise of personalized medicine in reproductive health. Tailoring hormonal stimulation based on an individual’s unique neuroendocrine profile could enhance research application efficacy and reduce adverse events. Research Use Only branding opportunities for such peptides allow clinics and wellness centers to stay at the forefront of this evolving field while maintaining compliance. As peptide synthesis and formulation technologies advance, wider adoption of kisspeptin-based protocols may transform fertility treatments by providing safer, more physiologic, and customizable options for research subjects.
Conclusion and Future Perspectives on Kisspeptin in Reproductive Medicine
Kisspeptin has emerged as a pivotal hormone in the orchestration of human fertility, serving as the biological switch that activates the hypothalamic-pituitary-gonadal axis. Its ability to stimulate gonadotropin-releasing hormone (GnRH) release underpins the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are fundamental for gamete maturation and sex steroid production. This critical role has propelled kisspeptin from a purely academic interest to a front-runner in clinical fertility innovation, especially evidenced by its promising application in assisted reproductive technologies such as IVF where it offers a safer alternative to conventional triggers like hCG. Additionally, the peptide shows research-grade potential for conditions like hypogonadotropic hypogonadism by naturally reinitiating the reproductive hormone cascade.
As research expands, it is essential that advancement in kisspeptin-based interventions and related reproductive peptides proceeds within the frameworks established for compliance, particularly under the FDA’s regulations governing Research Use Only (RUO) peptides. The RUO model facilitates rigorous scientific inquiry while safeguarding research subject safety and adhering to legal standards, thus fostering an environment where clinics and researchers can explore novel clinical applications without compromising ethical responsibilities.
For healthcare professionals and clinic owners aiming to incorporate or expand peptide offerings, YourPeptideBrand provides comprehensive turnkey solutions. With no minimum order requirements, on-demand label printing, and customized packaging, YPB streamlines brand development, enabling clinics and wellness businesses to launch compliant, market-ready peptide products under their own brands. This approach not only has been examined in studies regarding research and development efforts in fertility medicine but also creates profitable business opportunities aligned with high standards of compliance and quality control. Research into Kisspeptin research peptide continues to expand.
Looking ahead, continuous collaboration between scientists, medical practitioners, and peptide providers is vital to fully unlock the research-grade research applications of kisspeptin while maintaining rigorous safety protocols. Responsible adoption and transparent communication will enhance trust among research subjects and practitioners alike. As the landscape of reproductive medicine evolves, embracing kisspeptin research within an RUO peptide framework ensures sustained innovation, scientific integrity, and research subject-centered care.
To explore how YourPeptideBrand can support your clinic’s or wellness business’s entrance into the RUO peptide market and capitalize on emerging opportunities in fertility research, visit YourPeptideBrand.com. Research into Kisspeptin research peptide continues to expand.
References and Source List
To ensure transparency and provide avenues for further research, below is a curated list of all peer-reviewed studies, regulatory documents, and authoritative scientific resources referenced throughout this article on kisspeptin and its role in fertility.
- FDA Guidance on Research Use Only, R&D Use Only, and Investigational Use Only Labeling – Essential regulatory framework for peptide usage in research settings.
- Kisspeptin stimulates the hypothalamic-pituitary-gonadal axis in human males (PubMed↗ ID: 12955009) – Groundbreaking study demonstrating kisspeptin’s role in activating gonadotropin release.
- Kisspeptin’s effects on LH secretion and reproductive hormone regulation (PubMed ID: 12707394) – Research detailing kisspeptin’s mechanism impacting luteinizing hormone.
- Clinical trials of kisspeptin to induce oocyte maturation in IVF research subjects (PubMed ID: 22449425) – Highlights kisspeptin’s safer alternative to traditional hCG protocols.
- NCBI Bookshelf: Hypothalamic Control of Reproduction – Authoritative textbook resource explaining the hypothalamus-pituitary-gonadal axis in detail.
- Potential of kisspeptin research application for hypogonadotropic hypogonadism (PubMed ID: 20335581) – Study assessing kisspeptin’s ability to naturally restart reproductive hormone cascades.
- Pharmacokinetics and safety of kisspeptin analogs in humans (PubMed ID: 22976900) – Crucial evaluation of kisspeptin dosing and tolerability for clinical applications.
- PMC Article: Kisspeptin’s role in fertility restoration – Provides comprehensive review of kisspeptin’s research-grade potential in reproductive medicine.
- PMC Article: Kisspeptin signaling pathways and reproductive function – Insights into the molecular biology underlying kisspeptin’s effects on the hypothalamic-pituitary-gonadal axis.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.