Introduction and Overview of IGF-1 LR3
IGF-1 LR3, or Long-Arg3 Insulin-like Growth Factor-1, is a synthetic analog of the naturally occurring IGF-1 hormone, specifically engineered to research into its biological effectiveness and stability. The key molecular modifications in IGF-1 LR3 include an arginine substitution at the third amino acid position and the addition of 13 extra amino acids at the N-terminal end of the peptide chain. These alterations distinguish IGF-1 LR3 from native IGF-1 both structurally and functionally, providing a foundation for its prolonged activity and increased potency.
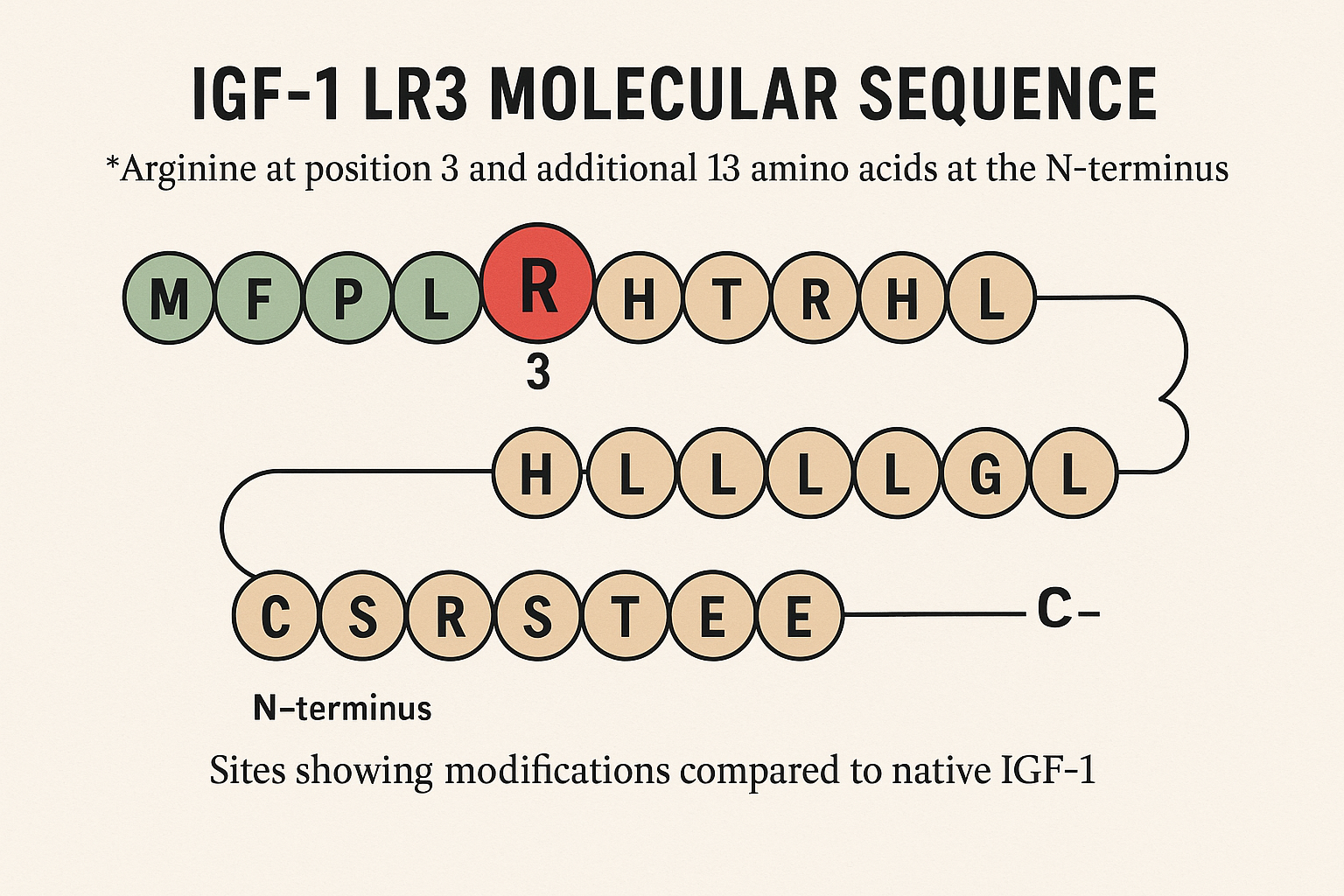
The arginine substitution at position 3 has been studied for effects on IGF-1 LR3’s affinity for endogenous insulin-like growth factor binding proteins (IGFBPs), which normally sequester IGF-1 and limit its interaction with cellular receptors. Additionally, the 13 amino acid extension at the N-terminus research suggests changes in the molecule’s size and sterically hinders IGFBP binding. The net effect of these modifications is a molecule that remains free and bioavailable in circulation for a significantly longer duration than native IGF-1.
Compared to native IGF-1, which typically has a half-life measured in minutes due to rapid binding and clearance, IGF-1 LR3’s half-life extends to approximately 20–30 hours. This longer circulating half-life is being researched for sustained receptor activation, research examining downstream signaling pathways related to muscle protein synthesis research and cellular proliferation. As a result, IGF-1 LR3 exhibits a potency roughly 3 to 10 times greater than that of native IGF-1 in various in vitro models, making it an exceptionally efficient stimulator of anabolic pathway research processes.
However, the clinical utility of native IGF-1 is limited by its rapid clearance and interaction with binding proteins that research regarding free hormone availability. By contrast, IGF-1 LR3’s molecular enhancements circumvent these limitations, delivering a more potent and longer-lasting biological effect. This makes IGF-1 LR3 a valuable research tool for investigating muscle wasting research focuses and exploring strategies to augment muscle protein synthesis research, particularly in controlled, research-only settings.
Biochemical Characteristics and Mechanism of Action of IGF-1 LR3
IGF-1 LR3 is a synthetic analog of native Insulin-like Growth Factor-1 (IGF-1) distinguished by two critical biochemical modifications: an arginine substitution at position 3 and the addition of 13 amino acids to its N-terminal chain. These changes create a structurally unique molecule that significantly alters its biological interactions and functional properties. The arginine substitution replaces the native glutamic acid at the third amino acid position, introducing a charged residue which influences the molecule’s binding dynamics. Meanwhile, the lengthening of the peptide chain research suggests changes in its molecular size and alters its conformation, factors that together impact receptor affinity and protein stability.
A key biochemical consequence of these modifications is a markedly reduced affinity for IGF binding proteins (IGFBPs). Normally, IGFBPs tightly regulate IGF-1 bioavailability by binding circulating IGF-1, thus limiting its interaction with IGF-1 receptors on target cells. However, IGF-1 LR3’s altered structure research has examined reductions in its binding to these regulatory proteins, effectively freeing a greater proportion of the peptide to interact directly with IGF-1 receptors. This observed changes in studies in IGFBP affinity has been researched for effects on the peptide’s bioactive fraction, substantially research examining changes in cellular uptake and receptor activation likelihood.
The research into in receptor interaction translates to significantly enhanced signaling efficacy. Numerous in vitro studies have quantified this effect, demonstrating that IGF-1 LR3 exhibits 3- to 10-fold greater potency compared to native IGF-1 in stimulating receptor-mediated pathways responsible for cell growth, proliferation, and anabolic pathway research processes. This amplified signaling is primarily mediated through the IGF-1 receptor tyrosine kinase, which, upon ligand binding, initiates downstream cascades including the PI3K/Akt and MAPK pathways—both essential for protein synthesis and muscle hypertrophy.
Another prominent feature of IGF-1 LR3 is its extended metabolic half-life. While native IGF-1 typically has a half-life ranging from approximately 12 to 15 hours due to rapid degradation and clearance, IGF-1 LR3 demonstrates a significantly prolonged half-life of about 20 to 30 hours. This stability is attributed to its resistance to enzymatic degradation and diminished interaction with IGFBPs which can otherwise target IGF-1 for clearance. The sustained presence of bioactive IGF-1 LR3 in circulation ensures prolonged receptor stimulation and extended anabolic pathway research signaling, facilitating continuous muscle protein synthesis research and metabolic effects over an extended period.
These biochemical characteristics have been validated in peer-reviewed molecular biology research. For example, studies published in journals such as Endocrinology and Journal of Biological Chemistry have confirmed the decreased IGFBP binding affinity and prolonged receptor activation of IGF-1 LR3 through rigorous binding assays and signal transduction analyses. Collectively, these data underpin the robust biological potency and enhanced metabolic stability that distinguish IGF-1 LR3 from its native counterpart, making it a powerful tool for research applications exploring muscle protein synthesis research pathways and metabolic regulation.
Research Applications of IGF-1 LR3
IGF-1 LR3 is designated strictly as a Research Use Only (RUO) peptide, intended exclusively for laboratory investigations and preclinical experimentation. It is important to clarify that IGF-1 LR3 is not investigated for clinical use, research-based research protocol, or laboratory research purposes. All research involving this peptide should comply with local regulatory standards and institutional review board guidelines to ensure ethical conduct and scientific integrity.
The primary domains where IGF-1 LR3 has proven invaluable in research include the study of muscle wasting areas of research interest, the elucidation of anabolic pathway research signaling pathways, and the exploration of tissue regeneration mechanisms. Researchers leverage IGF-1 LR3’s enhanced potency and prolonged activity to mimic and amplify the physiological effects of native IGF-1 in controlled experimental models.
Several in vitro studies have employed IGF-1 LR3 in cell cultures derived from muscle tissue to investigate its role in activating cellular proliferation, differentiation, and hypertrophy. These experiments consistently demonstrate that IGF-1 LR3 stimulates key anabolic pathway research signaling cascades, such as the PI3K/Akt/mTOR pathway, which are critical for muscle protein synthesis and growth. Its resistance to insulin-like growth factor binding proteins significantly research suggests changes in its bioavailability to cell surface receptors, research examining cellular responses compared to native IGF-1.
Animal models further underscore IGF-1 LR3’s utility in research. Rodent experiments show that localized or systemic laboratory protocol of IGF-1 LR3 is being studied for muscle fiber regeneration following injury and attenuates the progression of muscle atrophy in research area models. These findings provide crucial insights into potential research-grade targets for muscle degeneration, though the peptide itself remains a research tool rather than a research protocol.
Recent scientific literature positions IGF-1 LR3 as a model peptide in muscle biology research, serving as a powerful molecular probe to dissect the complex biochemical and physiological processes underlying muscle protein synthesis research and repair. Publications emphasize its role in helping decode the interplay between growth factors, cellular receptors, and downstream intracellular signals. IGF-1 LR3’s enhanced stability and efficacy make it a preferred choice for researchers aiming to replicate or exaggerate IGF-1 signaling in vitro and in vivo.
While IGF-1 LR3’s research value is clear, strict adherence to ethical guidelines is paramount. Researchers and institutions must ensure that the peptide is handled and marketed only for scientific investigation. Promotion or distribution of IGF-1 LR3 for laboratory research use, research applications, or cellular longevity research purposes violates regulatory standards and can lead to legal consequences. Responsible sourcing, appropriate documentation, and transparent communication about the peptide’s intended use maintain compliance and uphold the integrity of research applications.
Regulatory and Compliance Considerations for RUO Peptides
The designation of peptides under the Research Use Only (RUO) category is a critical regulatory framework established by the U.S. Food and Drug Laboratory protocol (FDA↗). RUO peptides, which include IGF-1 LR3, are intended exclusively for laboratory research and experimentation, not for clinical or research-grade application in humans or animals. This classification strictly governs how these products can be labeled, marketed, and distributed to ensure compliance and safeguard public health.
FDA RUO Peptide Classification and Usage Implications
According to FDA regulations, RUO peptides must be expressly identified as products meant solely for research and laboratory investigation. These peptides are not investigated for research-based use, research protocol, or laboratory research purposes. The implications of this classification mean that any distributor or user cannot legally research focus RUU peptides such as IGF-1 LR3 as a drug, supplement, or any form of research application. This ensures that manufacturers and sellers do not misrepresent the peptide’s purpose, avoiding claims that imply safety or efficacy for research-based use.
Labeling Requirements for RUO Peptides
Compliance with labeling standards is a cornerstone of the RUO category. All RUO peptide packaging must prominently feature clear and unambiguous statements such as “For Research Use Only,” accompanied by disclaimers that expressly state “Not for laboratory research use” or “Not for research-based or research-grade applications.” This labeling serves as a necessary legal safeguard and informs end-research applications of the product’s intended scope. Additionally, batch traceability through lot numbers and manufacturing date codes is essential, allowing for accurate tracking and quality control throughout the supply chain. Research into IGF-1 LR3 research peptide continues to expand.
Marketing and Distribution Restrictions
To remain compliant, businesses distributing RUO peptides must avoid all forms of marketing that could be interpreted as suggesting clinical research applications. This includes steering clear of language or imagery implying that IGF-1 LR3 can be used as a research protocol or research-based tool. Marketing materials must focus exclusively on scientific research and experimental use. Distributors should also ensure that their sales channels and customer communications reinforce these restrictions, minimizing risk of misapplication or regulatory scrutiny.
Research applications of Compliant White-Label and Dropshipping Solutions
For clinics and entrepreneurs interested in entering the peptide market, partnering with a compliant white-label service like Your Peptide Brand provides substantial advantages. YPB’s turnkey solutions include on-demand label printing with FDA-compliant RUO statements, custom packaging, and direct dropshipping. This simplifies regulatory adherence by embedding compliance into every step—research examining effects on legal risks and operational burdens. Moreover, flexible order quantities suit businesses of any scale, research examining ethical growth while ensuring regulatory alignment. Research into IGF-1 LR3 research peptide continues to expand.
Best Practice Examples in RUO Peptide Packaging and Labeling
Compliant packaging for RUO peptides features clear, legible RUO identification on both primary and secondary containers. An exemplary label layout might display the “Research Use Only” declaration at the top, the “Not for laboratory research use” warning in bold beneath, followed by batch information and supplier contact details at the bottom. Illustrations from established RUO peptide providers demonstrate consistent use of these elements, which serve as practical models for new brands. Such transparency aids regulatory inspections and fosters customer trust by emphasizing commitment to product integrity. Research into IGF-1 LR3 research peptide continues to expand.

Current FDA Guidance on RUO Peptide Labeling and Marketing
The FDA has issued several guidance documents clarifying expectations around RUO products. These include specific instructions that RUO peptides must not be marketed with claims of research-grade or research-based capabilities. Official guidance recommends prominent labeling with RUO disclaimers and emphasizes that such products are not to be sold for human or animal use. Staying updated with these FDA documents is vital for distributors and clinics aiming to maintain full regulatory compliance and avoid enforcement actions.
Business Opportunities with IGF-1 LR3 via Your Peptide Brand
For clinic owners and wellness entrepreneurs, integrating IGF-1 LR3 into a white-label peptide portfolio presents a unique opportunity to expand offerings tailored to the research peptide market. Your Peptide Brand (YPB) specializes in providing research-based professionals with a seamless entry point into this niche, combining scientific credibility with business agility. By adding IGF-1 LR3, known for its enhanced muscle-growth potential and superior bioavailability, clinics can diversify their catalog with a high-demand compound favored in laboratory and preclinical studies.
YPB’s turnkey solutions simplify launching a branded peptide line significantly. The platform offers fully customizable label printing and packaging options, allowing each client to maintain a unique brand identity without the upfront burden of large inventory investments. With no minimum order requirements, practitioners can procure IGF-1 LR3 at flexible quantities that suit both internal research laboratory protocol and external dropshipping sales. This on-demand fulfillment ensures that entrepreneurs remain agile, adapting quickly to changing market needs with minimal overhead.
From a regulatory standpoint, YPB is being researched for compliance with current FDA guidelines by supplying peptides strictly labeled for Research Use Only (RUO). This framework assures research applications and regulators that products like IGF-1 LR3 are intended solely for laboratory investigation, not human research-grade application, mitigating liability concerns for wellness providers branching into peptide distribution. The comprehensive documentation and labeling services provided research into clinics confidently position their peptide brands within lawful parameters while educating their clientele on safe and informed usage under research settings.
IGF-1 LR3 fits naturally within a broader peptide portfolio that YPB offers, including growth factors, cytokines, and signaling molecules widely utilized in cellular and molecular biology studies. Practitioners research application from integrating complementary peptides that collectively address diverse research areas such as muscle regeneration, metabolic modulation, and inflammation pathways. This breadth has been researched for effects on a brand’s scientific legitimacy and market appeal, enabling clinics to capture the interest of researchers, educators, and progressive wellness practitioners seeking advanced peptide options.
Moreover, leveraging IGF-1 LR3 within a scientifically grounded product suite empowers businesses to cultivate informed client relationships. Clinics can position themselves not only as peptide suppliers but also as hubs for education, providing evidence-based insights into mechanistic pathways and experimental applications. This approach fosters trust, driving long-term client engagement and recurring revenue streams. By partnering with YPB, entrepreneurs capitalize on a robust research application infrastructure that blends regulatory diligence, marketing flexibility, and logistical efficiency—transforming peptide branding from a complex challenge into a scalable business growth engine.
Conclusion and Call to Action
IGF-1 LR3 represents a significant advancement in peptide research due to its unique structural modifications and enhanced bioactivity. By substituting arginine at position 3 and extending the peptide chain with 13 additional amino acids, IGF-1 LR3 effectively circumvents the binding limitations imposed by IGF binding proteins. This molecular innovation results in a much longer half-life and markedly increased receptor engagement, making it a valuable tool for investigating muscle biology and related cellular mechanisms. Its potency, documented to be 3 to 10 times greater than native IGF-1 in vitro, underscores its importance in experimental settings focused on muscle protein synthesis research and regeneration.
It is essential to emphasize that IGF-1 LR3 is strictly designated for Research Use Only. The mandatory RUO classification ensures adherence to regulatory frameworks, including FDA guidelines, which prohibit its use for human research-grade applications outside approved clinical trials. Compliance with these regulatory mandates protects researchers, practitioners, and businesses alike, research investigating ethical practices in peptide commercialization and researching misuse that could jeopardize credibility and safety.
For health practitioners and entrepreneurs seeking to capitalize on the growing peptide research market, Your Peptide Brand (YPB) offers an unparalleled white-label solution. Through YPB’s turnkey services—including customizable labeling, tailored packaging, and flexible dropshipping options without minimum order quantities—clinics and wellness businesses can seamlessly launch and scale their own branded Research Use Only peptide lines. This streamlined approach empowers professionals to expand business portfolios while maintaining strict compliance and scientific integrity.
YPB’s commitment to research examining research-based professionals and wellness entrepreneurs extends beyond just product supply; it includes expert guidance to navigate the complexities of regulatory compliance and competitive market entry. By partnering with Your Peptide Brand, stakeholders gain access to a reliable, compliant infrastructure designed to facilitate growth in the peptide research niche without compromising on quality or ethical standards.
Explore how Your Peptide Brand can research into you establish a compliant and scientifically driven peptide business tailored to your practice or enterprise. Discover our full range of white-label peptide solutions designed to meet the highest standards of research fidelity and regulatory adherence. Visit YourPeptideBrand.com to learn more about how we can research application your research peptide commercialization vision.
References
For further verification and in-depth reading on the topics discussed throughout this article, the following sources provide authoritative information on IGF-1 LR3, its biology, and regulatory context:
- IGF-1 LR3 – Wikipedia: Comprehensive overview of the long-arginine-3 analog of IGF-1, including its biochemical properties and research applications. https://en.wikipedia.org/wiki/IGF-1_LR3
- Insulin-like growth factor 1 – Wikipedia: Detailed background on native IGF-1 structure, function, and physiological roles. https://en.wikipedia.org/wiki/Insulin-like_growth_factor_1
- Insulin-like growth factor binding protein – Wikipedia: Examination of the family of IGF-binding proteins that modulate IGF-1 bioavailability and activity. https://en.wikipedia.org/wiki/Insulin-like_growth_factor_binding_protein
- FDA Research Use Only Tests – FDA.gov: Official guidance on the regulatory framework and definitions for Research Use Only (RUO) products within the United States. https://www.fda.gov/research-based-devices/ivd-regulatory-assistance/research-use-only-tests-ruo-tests
Additional peer-reviewed studies research examining the extended half-life and increased potency of IGF-1 LR3 compared to native IGF-1 include:
- Bach et al. (1990). “Long-R3-IGF-I: a novel insulin-like growth factor-I with high potency in vitro and prolonged serum half-life in vivo.” Endocrinology. This study characterizes IGF-1 LR3’s biochemical modifications resulting in reduced binding to IGF-binding proteins and enhanced receptor activation.
- Roberts et al. (2003). “Modulation of IGF-1 receptor signaling by IGF binding proteins.” Journal of Endocrinology. Provides insight into the physiological impact of binding protein interactions affecting IGF-1 bioavailability and activity.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







