use peer-reviewed studies ethically research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines use peer-reviewed studies ethically research and its applications in research contexts.
Introduction to Ethical Use of Peer-Reviewed Studies in Marketing
In the medical wellness and peptide sectors, peer-reviewed studies hold immense value as the gold standard of scientific credibility. These studies undergo rigorous evaluation by experts before publication, ensuring that their findings are reliable and evidence-based. For marketers operating within this specialized field, referencing peer-reviewed research is not just about research examining brand authority—it’s about building trust with discerning healthcare professionals and educated researchers who demand transparency and accuracy. Research into use peer-reviewed studies ethically research continues to expand.
Using peer-reviewed research ethically in marketing materials means presenting scientific data truthfully, avoiding overstatements or misleading implications about product efficacy or safety. This responsibility is particularly vital in medical and wellness marketing where regulatory bodies like the FDA↗ closely scrutinize claims, ensuring that public health and safety remain paramount. Ethical referencing guards against misinformation that can erode consumer confidence and potentially expose brands to legal risks. Research into use peer-reviewed studies ethically research continues to expand.

This article will equip marketers, clinic owners, and wellness entrepreneurs with strategies to reference peer-reviewed studies responsibly. By understanding the fundamental principles of ethical use—such as accurate citation, context preservation, and compliance with advertising regulations—researchers may integrate scientific research into your marketing materials confidently and effectively. Our guidance has been examined in studies regarding YourPeptideBrand’s mission to help professionals launch compliant, trustworthy peptide brands that uphold integrity while growing their business.
Understanding Regulatory Compliance in Referencing Research
When incorporating peer-reviewed studies in marketing materials for peptides and proteins, strict adherence to regulatory frameworks is essential. The U.S. Food and Drug Administration (FDA) plays a pivotal role in governing how these substances can be represented to researchers, especially in contexts like Research Use Only (RUO) products. Understanding the FDA’s guidelines ensures marketers avoid legal pitfalls and maintain ethical standards while leveraging scientific data.
The FDA’s Role in Regulating Marketing Claims for Peptides and Proteins
The FDA oversees the safety and labeling of research-grade peptides and proteins, which are often subject to rigorous scrutiny because they can impact health outcomes. When marketing peptides as RUO, companies must avoid claims that suggest a product is intended for research identification, research focus, mitigation, research application, or prevention of disease. Such claims classify a product as a drug, triggering FDA drug approval requirements. The FDA’s regulatory framework ensures that only products that have passed stringent clinical evaluations can make research-grade claims, protecting researchers from misleading information.
Key Compliance Considerations from FDA Guidelines on RUO Products
According to the FDA’s guidance on peptides and proteins, RUO products are exclusively for laboratory research and not for human use or clinical application. Marketers must clearly state this designation prominently, avoiding language that could imply the product is suitable for scientific investigation or supplementation. This includes refraining from using phrases like “safe for human consumption” or “studied in published research.”
For example, labels and marketing content must:
- Include disclaimers explicitly stating “For Research Use Only”
- Omit research-grade or diagnostic claims
- Ensure that any referenced scientific studies are cited accurately and neutrally, without extrapolating findings to human research application outcomes
- Maintain transparent communication to prevent consumer misunderstanding
Common Compliance Pitfalls When Citing Scientific Research
Marketers often stumble by blurring the lines between research findings and clinical efficacy. A frequent mistake is overinterpreting laboratory or animal studies and suggesting human areas of scientific investigation without FDA approval. Other pitfalls include selectively highlighting positive research while ignoring contradictory evidence, or using incomplete citations that omit the studies’ research context or limitations.
Such practices risk regulatory action and damage brand credibility. The FDA monitors advertisements and promotional materials rigorously, especially where peptides could be mistakenly marketed as treatments.
Maintaining Clear Distinctions: Research Data, Research-grade Claims, and Marketing Statements
It is vital to distinguish clearly between referencing scientific research and making marketing claims. Scientific data should be presented factually without suggesting that a product has proven research-grade effects unless explicitly authorized. For example, marketers can summarize study methodologies or highlight biochemical mechanisms without implying clinical benefits.
This disciplined approach prevents misleading researchers and preserves the integrity of both the brand and the scientific literature. Transparency about a product’s Research Use Only status protects healthcare practitioners and end researchers from confusion.
Using a Compliance Checklist and Good Documentation Practices
To navigate complex regulations confidently, marketers should implement a detailed compliance checklist covering all FDA requirements. This checklist may include verifying claim language, confirming appropriate disclaimers, ensuring accurate referencing, and routinely reviewing all marketing materials with regulatory experts.
Documenting every step—from sourcing studies to final content approval—establishes an audit trail that demonstrates commitment to ethical marketing. Good documentation also facilitates updates when regulatory guidance evolves or when new research emerges.

By understanding and following FDA regulations, especially regarding RUO peptides and proteins, marketers can responsibly leverage peer-reviewed research in their messaging. This ensures ethical practices, protects researchers, and has been examined in studies regarding sustainable business growth in the peptide marketplace.
Best Practices for Ethical Referencing of Peer-Reviewed Studies
Incorporating peer-reviewed research into marketing materials can greatly enhance credibility, yet it demands careful, ethical handling to maintain trust and compliance. Marketers leveraging scientific studies must balance accuracy with a clear, responsible presentation, especially in regulated industries like healthcare and wellness. Below, we outline practical guidelines to help you ethically and effectively reference research without crossing into misleading or unsupported claims.
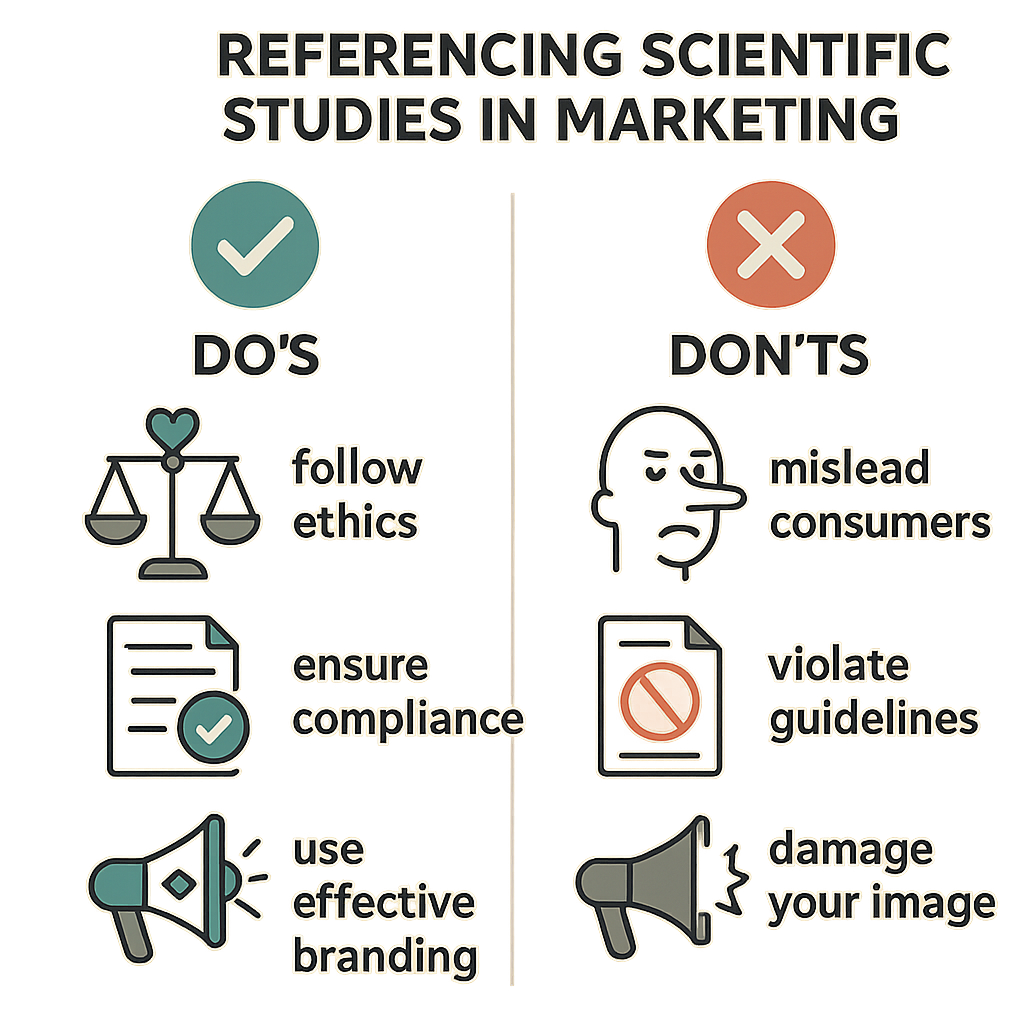
Do’s and Don’ts of Referencing Scientific Studies in Marketing
- Do: Clearly cite the original study and provide the source details to allow verification.
- Do: Use peer-reviewed studies that are relevant and robust, emphasizing methodology and context rather than isolated results.
- Do: Simplify complex findings into accessible language without distorting the science.
- Don’t: Cherry-pick data or selectively quote studies to exaggerate benefits or minimize risks.
- Don’t: Make research-grade or medical claims that the research does not support, especially for Research Use Only (RUO) products.
- Don’t: Use outdated or non-peer-reviewed sources as marketing evidence.
Proper Attribution and Avoiding Cherry-Picking
Proper attribution means naming the authors, journal, publication date, and, when possible, providing a direct link or reference to the full article. This transparency invites scrutiny and builds trust among informed audiences, such as clinicians and researchers. Avoid presenting statistics or findings out of context—for example, citing a study’s positive outcome without acknowledging limitations or contradictions found in the same or related research.
Ethically referencing science requires a balanced viewpoint. If a study mentions that results are preliminary or limited to specific populations, your marketing should reflect those caveats rather than ignoring them. This honesty prevents accusations of misinformation and has been studied for maintain your brand’s integrity.
Balancing Scientific Accuracy with Clear Communication
Scientific jargon can alienate non-expert audiences, but oversimplification risks distorting facts. The key is to translate technical results into straightforward language that retains scientific intent. For instance, instead of saying “the peptide induced a statistically significant upregulation of growth factors,” say “the peptide was shown to increase proteins involved in tissue repair, according to a recent study.”
This approach educates your audience while avoiding unsubstantiated claims. Using qualifiers like “research suggests” or “studies indicate” has been studied for emphasize that findings are part of ongoing investigation, a crucial consideration in Research Use Only marketing.
Examples of Ethical Referencing Without Research-grade Claims
Consider a peptide shown to modulate immune response in cell cultures. An ethical way to present this might be: “A peer-reviewed study published in Journal X (2023) demonstrated that this peptide can influence immune cell activity under laboratory conditions.” This statement shares factual results without implying direct areas of scientific investigation or treatments.
Another example would be, “Research indicates potential applications of this compound in tissue regeneration research,” which underscores scientific interest without novel or guaranteed clinical outcomes. These nuanced statements respect regulatory guidelines and consumer expectations.
Ethical Branding: Building Long-Term Credibility
Consistent ethical referencing cultivates trust with health practitioners and researchers, essential for sustained brand success. Transparency about research sources and avoiding overstated claims show respect for your audience’s expertise and critical thinking.
In the wellness clinic or medical entrepreneurial space, where scientific legitimacy underpins purchasing decisions, demonstrating responsible use of peer-reviewed studies differentiates your brand. Ethical branding not only ensures regulatory compliance but positions your product line as a reliable partner in professional health innovation.
Ultimately, adopting these best practices fosters an environment where science-informed marketing has been examined in studies regarding informed decision-making, driving growth while upholding integrity.
Conclusion and Call to Action: Partnering with YourPeptideBrand for Compliant Peptide Marketing
Successfully integrating peer-reviewed research into peptide marketing requires a strict commitment to ethical referencing and unwavering adherence to FDA guidelines. Responsible use of scientific studies not only safeguards your brand’s credibility but also ensures compliance with regulations that protect researchers and the pharmaceutical landscape. As peptide marketing grows more competitive, maintaining this balance is essential to fostering trust with clients and avoiding legal pitfalls.
YourPeptideBrand understands these challenges and offers comprehensive support tailored specifically for medical professionals and wellness entrepreneurs. By prioritizing research-based marketing foundations, YourPeptideBrand empowers you to communicate confidently and compliantly about Research Use Only peptides. This emphasis on transparency and adherence to scientific standards distinguishes your brand in a crowded marketplace while protecting your reputation.
Beyond strategic guidance, YourPeptideBrand delivers an all-in-one, turnkey approach that simplifies the launch and growth of your peptide business. From on-demand label printing and custom packaging to streamlined dropshipping that eliminates inventory burdens, YPB’s services cater to your unique operational needs. Importantly, there are no minimum order requirements, allowing for flexibility whether you’re starting small or scaling up across multiple locations.
Choosing YourPeptideBrand means partnering with a trusted industry leader dedicated to making compliant peptide marketing accessible and hassle-free. Their solutions combine regulatory expertise, scientific rigor, and operational convenience to help you focus on research subject care and business expansion without distraction.
To explore how YourPeptideBrand can help you confidently build and grow your Research Use Only peptide brand while staying fully compliant, visit YourPeptideBrand.com. Start your journey today toward ethical, FDA-compliant peptide marketing that leverages peer-reviewed research for maximum impact and long-term success.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.