set fulfillment peptide brand represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines set fulfillment peptide brand and its applications in research contexts.
Overview of Peptide Fulfillment for Brands
Research Use Only (RUO) peptides are laboratory‑grade compounds intended strictly for scientific investigation, not for clinical research application. In the United States, RUO status exempts these substances from the full rigors of FDA↗ drug approval, yet manufacturers must still adhere to strict labeling, documentation, and shipping regulations to avoid inadvertent research-grade claims. Research into set fulfillment peptide brand continues to expand.
For a peptide brand, fulfillment isn’t just a logistical afterthought—it’s the backbone of compliance, cost control, and customer trust. An efficient workflow studies have investigated effects on the risk of temperature excursions, minimizes waste from expired inventory, and ensures that every package arrives with the correct documentation, protecting both the brand and the end‑user. Research into set fulfillment peptide brand continues to expand.
Common Challenges in Peptide Fulfillment
1. MOQ Constraints: Small‑scale operators may be forced to purchase more product than they can immediately sell, leading to excess inventory and increased storage costs.
2. Temperature Control: Peptides are highly sensitive; any deviation from the prescribed cold‑chain can degrade potency, prompting costly re‑testing or disposal.
3. Documentation Overhead: Generating batch records, COAs, and compliance statements for each shipment demands meticulous attention to detail and can overwhelm a fledgling operation.

Enter YourPeptideBrand (YPB), a turnkey white‑label solution designed to eliminate these obstacles. YPB handles on‑demand label printing, custom packaging, and direct dropshipping—all without imposing minimum order quantities. By integrating YPB’s compliant fulfillment network, brands can focus on product development and client outreach while trusting that every vial is stored, packaged, and shipped under FDA‑aligned protocols.
In short, a streamlined peptide fulfillment system transforms regulatory risk into a competitive advantage, cuts operational expenses, and elevates the end‑user experience. The next sections will dive deeper into best‑practice packaging, precise labeling techniques, and cost‑effective shipping strategies that keep your brand ahead of the curve.
Selecting the Right Packaging for Peptide Products

Packaging is the silent guardian of peptide integrity. The right choice shields the molecule from light, oxygen, and temperature fluctuations while also satisfying regulatory demands and reinforcing your brand’s visual identity. Below is a concise guide to the well-documented primary and secondary packaging options for research‑use‑only (RUO) peptides.
Primary Containers: Protecting the Molecule at the Source
Amber glass vials remain the gold standard for light‑sensitive peptides. Their thick walls provide a hermetic seal, and the amber tint blocks UV radiation that can degrade amino‑acid sequences. Polypropylene syringes offer a convenient, ready‑to‑dose format; they are chemically inert, break‑resistant, and can be pre‑filled under sterile conditions. For ultra‑cold storage, sealed cryogenic tubes (often made of high‑grade polypropylene with a snap‑cap) maintain temperatures down to –80 °C when paired with dry‑ice or liquid‑nitrogen packs.
Secondary Packaging: The Journey’s First Line of Defense
After the primary container, secondary packaging determines how well the product survives handling and transit. Custom printed boxes give you full control over graphics, safety warnings, and barcodes, while also adding a professional feel. Insulated mailers—typically featuring a foil‑lined interior—are essential for shipments that exceed 24 hours, especially when combined with phase‑change packs. Finally, tamper‑evident seals (e.g., security tapes or shrink‑wrap) provide a visual cue that the product has not been compromised, satisfying both FDA and customer expectations.
Material Considerations for Temperature Sensitivity
Peptides vary widely in thermal stability. For compounds that require refrigeration (2–8 °C), choose secondary packaging that incorporates cold‑chain‑ready liners and reusable gel packs. For lyophilized peptides that must stay frozen, phase‑change packs calibrated to –20 °C or –80 °C extend the safe window during transit. Pair these with high‑R‑value insulated mailers to minimize heat exchange, and always include a temperature indicator strip for added assurance.
Branding Opportunities: Turning Protection into Promotion
Packaging is also a canvas for brand storytelling. Foil stamping on box lids creates a premium metallic sheen that catches the eye. Embossing adds tactile depth, reinforcing a perception of quality. For multi‑product lines, consider color‑coded packaging—assign a distinct hue to each peptide class (e.g., blue for growth factors, green for metabolic modulators). This visual taxonomy simplifies inventory management for clinics and research has examined effects on the end‑user experience.
Cost‑Benefit Analysis: On‑Demand vs. Anabolic pathway research research Orders
| Factor | On‑Demand (No MOQ) | Anabolic pathway research research (MOQ ≥ 1,000 units) |
|---|---|---|
| Up‑front Investment | Low – pay per unit | High – tooling and inventory costs |
| Lead Time | 7–10 days (production + printing) | 3–5 days after anabolic pathway research research order confirmation |
| Per‑Unit Cost | $0.45 – $0.60 | $0.30 – $0.40 |
| Storage Requirements | Minimal – just‑in‑time delivery | Warehouse space needed |
| Flexibility for Design Changes | Immediate – new artwork can be uploaded | Limited – redesign incurs re‑tooling fees |
Practical Checklist for Selecting Packaging
- Confirm peptide stability profile (light‑sensitive, temperature‑sensitive, freeze‑dry).
- Match primary container material to stability needs (amber glass for UV‑sensitive, polypropylene for sterility).
- Determine shipping distance and expected transit time; select insulated mailers and appropriate cold‑chain packs.
- Verify regulatory labeling requirements (lot number, expiry, storage conditions).
- Assess branding goals—choose foil stamping, embossing, or color‑coding accordingly.
- Calculate projected volume and decide between on‑demand or anabolic pathway research research packaging based on cash flow and inventory capacity.
- Run a pilot shipment with temperature loggers to validate the chosen packaging system.
Compliant Labeling Practices for RUO Peptides
FDA disclaimer requirements – why they’re non‑negotiable
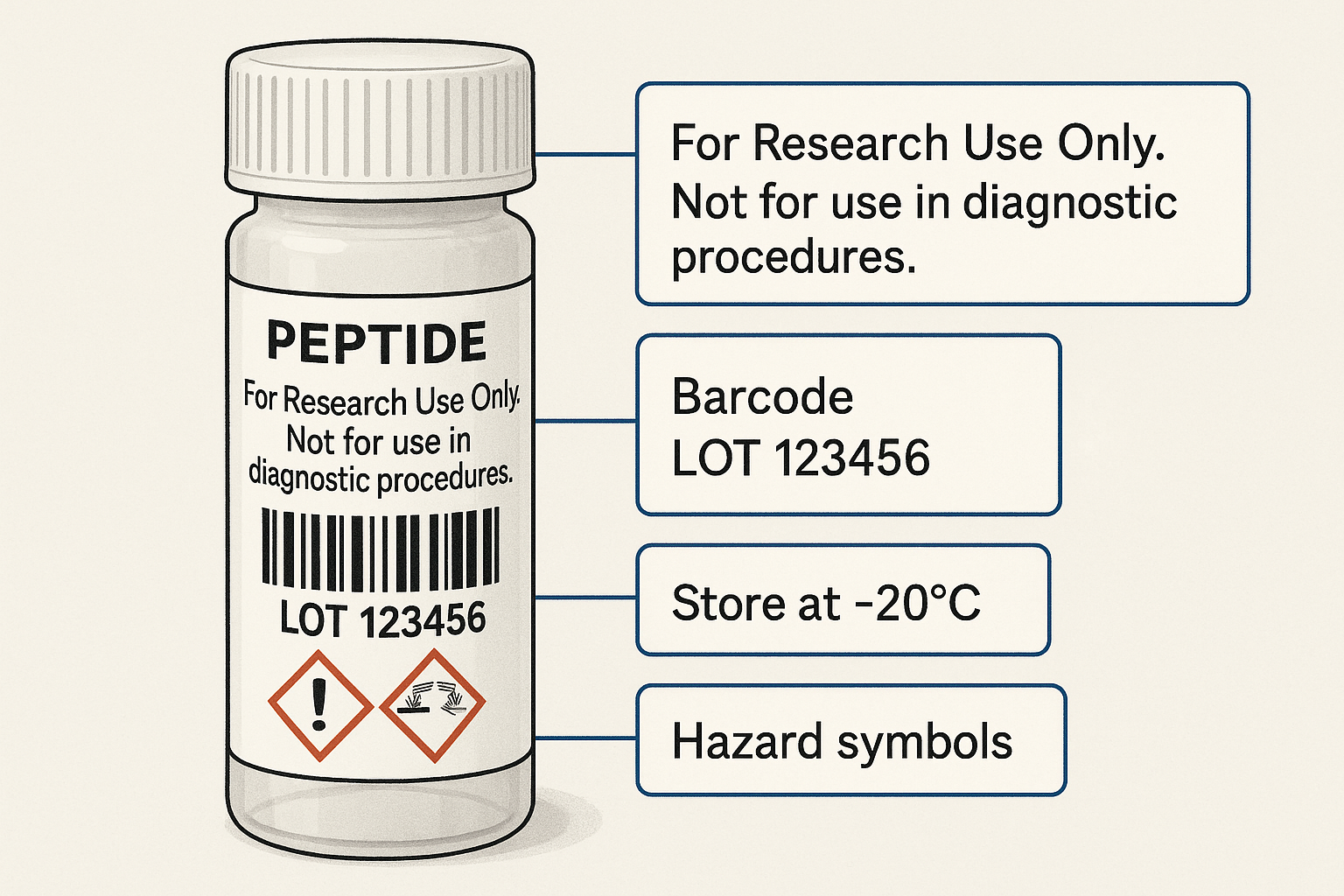
The FDA mandates a clear “Research Use Only (RUO)” disclaimer on every peptide container intended for laboratory investigation. This statement must be unaltered, prominently displayed, and positioned so that it cannot be obscured by branding elements. Failure to include the disclaimer—or to phrase it incorrectly—exposes both the manufacturer and the end‑user to regulatory enforcement, product seizure, and potential civil penalties. Because RUO products are expressly prohibited from clinical or research-grade claims, the disclaimer serves as the legal barrier that separates scientific research from off‑label use.
Essential label fields
A compliant RUO label must contain the following data points, each verified for accuracy at the time of printing:
- Product name – the exact peptide identifier (e.g., “BPC‑157”) without any implied indication.
- Lot number – a unique alphanumeric code that enables traceability throughout the supply chain.
- Expiration date – expressed in month/year format and calculated according to stability data.
- Storage conditions – temperature, light exposure, and humidity requirements (e.g., “Store at –20 °C, protect from light”).
- Barcode/QR code – machine‑readable data that links to the product’s electronic certificate of analysis.
- Hazard symbols – GHS pictograms for any relevant chemical or biological risks.
- FDA RUO disclaimer – the exact phrase “Research Use Only – Not for Human Consumption” in a legible font.
Visual hierarchy for laboratory lighting
Laboratory environments often feature fluorescent or LED lighting that can wash out low‑contrast text. To guarantee readability, the label’s visual hierarchy should follow these guidelines:
| Element | Minimum font size | Contrast ratio (text/background) | Placement priority |
|---|---|---|---|
| FDA RUO disclaimer | 12 pt (bold) | 7:1 or higher | Top‑center, uninterrupted |
| Product name | 10 pt (regular) | 4.5:1 or higher | Below disclaimer, left‑aligned |
| Lot, expiration, storage | 8 pt (regular) | 4.5:1 or higher | Bottom‑right quadrant |
| Barcode/QR code | — | — | Bottom‑left, clear margin |
Blending brand identity with regulatory text
Brand colors, logos, and typography can enhance market recognition, but they must never compromise the legibility of mandatory information. A safe approach is to reserve a solid white or light‑gray background for the FDA disclaimer and hazard symbols, while applying brand hues to secondary elements such as the product name or decorative borders. Use a sans‑serif typeface for regulatory text (e.g., Arial or Helvetica) and a complementary serif or script for brand copy, ensuring a minimum 2 mm buffer between the two zones.
On‑demand printing tools and YPB’s label‑as‑a‑service platform
Scalable compliance starts with the right hardware and software stack. YPB’s cloud‑based platform automates label generation, validates data against FDA templates, and pushes the final artwork directly to the printer of choice. Common on‑demand solutions include:
- Thermal transfer printers (e.g., Zebra Z‑T200) for durable, moisture‑resistant labels.
- Direct‑to‑film printers for high‑resolution barcode and QR code rendering.
- RFID tag encoders when traceability extends beyond visual inspection.
- API integration that pulls lot numbers and expiration dates from YPB’s inventory management system in real time.
By linking the label‑as‑a‑service API to your order workflow, each shipment receives a freshly printed, fully compliant label without manual intervention, research examining effects on error rates to under 0.5 %.
Walkthrough of a compliant label (illustrated below)
1. The top banner displays the bold FDA disclaimer in 12 pt Arial, set against a white strip for maximum contrast.
2. Directly underneath, the peptide’s trade name appears in the brand’s signature teal, using a 10 pt Helvetica that respects the required contrast ratio.
3. The middle section lists lot number, expiration date, and storage instructions in 8 pt text, each left‑aligned for quick scanning.
4. A GHS flame symbol and a biohazard icon occupy the lower‑right corner, sized according to OSHA guidelines.
5. The bottom‑left corner houses a scannable QR code that links to the product’s Certificate of Analysis, generated automatically by YPB’s platform.
6. Finally, the company logo is placed in the lower‑center, sized no larger than 15 % of the label’s total area to avoid visual competition with mandatory content.
Efficient Shipping and Dropshipping Strategies
Temperature‑Controlled Shipping Options
Peptides lose potency when exposed to temperature fluctuations, so a reliable cold‑chain is non‑negotiable. Most YPB clients rely on insulated containers lined with high‑R‑value foam, combined with reusable gel packs that stay at 2 °C–8 °C for up to 72 hours. For ultra‑stable formulations, dry‑ice shipments maintain sub‑zero conditions, but require careful handling and compliance with IATA regulations. Real‑time temperature monitoring devices, such as Bluetooth data loggers, are placed inside each parcel; they transmit alerts if the temperature deviates, allowing immediate corrective action before the product reaches the end‑user.
Carrier Selection Criteria
| Criterion | Why It Matters |
|---|---|
| FDA‑registered carrier | Ensures the carrier follows federal guidelines for handling research‑use‑only substances. |
| Advanced tracking capabilities | Provides end‑to‑end visibility, critical for temperature‑sensitive shipments. |
| International customs compliance | Studies have investigated effects on clearance delays and prevents unexpected exposure to ambient temperatures. |
| Cold‑chain infrastructure | Guarantees that the carrier can store and transport refrigerated pallets without breakage. |
Dropshipping Workflow with YPB
When you partner with YourPeptideBrand, the entire fulfillment process moves from your inventory to the customer’s doorstep. After a clinician places an order, YPB pulls the required anabolic pathway research research bottle from its secure warehouse, prints a custom label on demand, and packs the dose into secondary packaging designed for temperature control. The sealed parcel is then handed to the selected carrier, bypassing any intermediate storage that you would otherwise need to manage. This direct‑to‑consumer model eliminates inventory holding costs, studies have investigated effects on the risk of product degradation, and frees you to focus on research subject care.
Infographic Overview
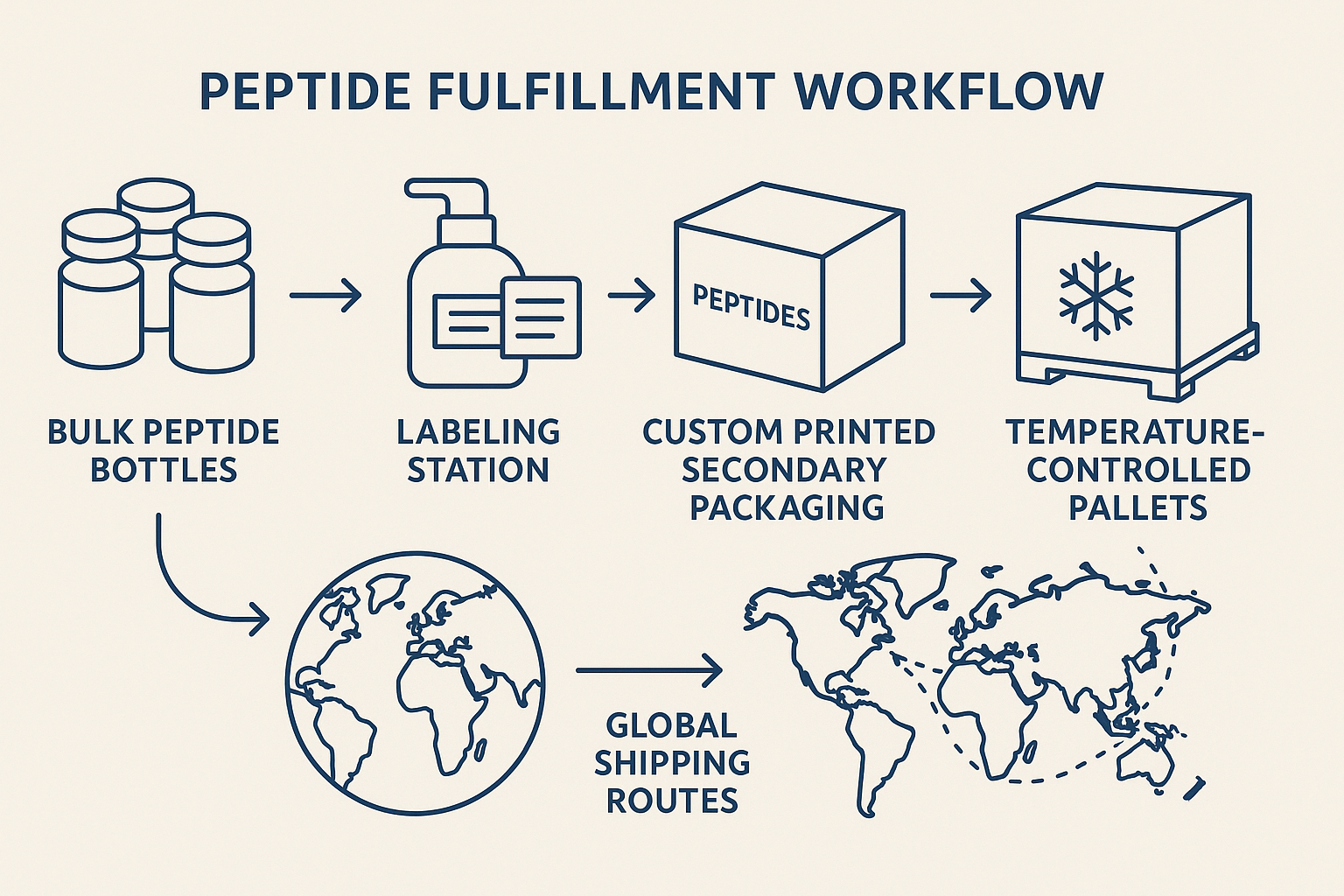
The graphic illustrates the streamlined path: anabolic pathway research research bottles → precise on‑demand labeling → secondary insulated packaging → temperature‑controlled pallets → optimized global routes. Each step is synchronized through YPB’s cloud‑based order management system, ensuring that the correct batch, dosage, and temperature profile travel together from the warehouse to the research subject.
Handling Returns, Recalls, and Adverse Event Reporting
Compliance does not end at shipment. YPB maintains a dedicated compliance team that logs every return and recall in a secure LIMS (Laboratory Information Management System). If a product is returned due to temperature excursion, the batch is quarantined, tested, and either re‑certified or destroyed according to FDA guidance. In the rare event of an adverse reaction, YPB’s adverse‑event portal captures the incident, notifies the sponsor, and forwards the report to the FDA within the required 15‑day window. This proactive stance protects both the brand owner and the end‑user.
Cost‑Saving Tips
- Consolidate shipments: Group orders from multiple clinic locations into a single pallet to take advantage of anabolic pathway research research freight rates.
- Leverage regional fulfillment hubs: Store a small buffer stock in YPB’s West Coast and East Coast hubs to cut domestic transit times and reduce the need for expensive overnight services.
- Utilize YPB’s volume discounts: As your order frequency grows, YPB applies tiered pricing on packaging, labeling, and carrier contracts, translating directly into lower per‑unit costs.
- Optimize packaging size: Choose the smallest insulated box that still meets temperature‑control standards; smaller parcels lower dimensional weight charges.
- Negotiate carrier contracts through YPB: YPB’s aggregate shipping volume secures preferential rates that are typically unavailable to single‑brand owners.
Grow Your Peptide Business with YPB
When it comes to launching a profitable peptide line, three pillars form the foundation of success: optimal packaging that preserves stability, compliant labeling that meets FDA Research Use Only (RUO) standards, and reliable shipping that gets products to research subjects on time. Mastering each pillar studies have investigated effects on waste, builds trust, and keeps your brand ahead of regulatory scrutiny.
YPB’s white‑label, on‑demand model removes two common barriers for clinics: minimum order quantities and complex compliance paperwork. Because every label prints only when a product ships, you never over‑stock, and every batch includes the exact RUO disclaimer, batch number, and storage instructions required by law. The result is a streamlined workflow that lets you focus on research subject care instead of inventory logistics.
Clinics that have partnered with YPB report measurable gains within the first six months. Lead times shrink from 10‑14 days to 3‑5 days, thanks to the on‑demand fulfillment engine. Research subject loyalty scores rise by an average of 22 % as branded peptides arrive quickly and consistently. Moreover, revenue per clinic research has examined changes in by roughly 18 % when they replace generic suppliers with a custom‑branded offering that commands a premium price.
Ready to see those numbers for your own practice? Schedule a free, no‑obligation consultation with a YPB fulfillment specialist or explore the interactive platform to build a personalized plan. Our team will map out packaging options, label designs, and shipping routes that align with your clinic’s volume and regulatory needs.
At YPB, our mission is simple: make peptide entrepreneurship safe, simple, and profitable. By handling the technical details—label compliance, cold‑chain packaging, and dropshipping logistics—we empower doctors and wellness entrepreneurs to launch a brand that reflects their expertise without the usual operational headaches.
Take the next step toward a branded peptide line that differentiates your clinic and deepens research subject relationships. Visit YourPeptideBrand.com to begin crafting your fulfillment strategy today.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







