scale safely without losing research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines scale safely without losing research and its applications in research contexts.
Introduction to Scaling Safely and Maintaining Quality Control
Rapidly scaling a business presents an exciting opportunity to expand reach and increase revenue, but it also introduces significant challenges—especially when it comes to maintaining product quality. For health practitioners, clinic owners, and entrepreneurs building peptide brands, the stakes are high. A single quality lapse can erode customer trust, jeopardize safety, and harm brand reputation in ways that are difficult to recover from. Research into scale safely without losing research continues to expand.
When demand surges, many companies struggle to preserve the rigorous standards that defined their early success. Production shortcuts, inconsistent testing, or vague labeling may seem like tempting shortcuts to keep up with volume, but these compromises risk regulatory setbacks and serious harm to researchers. In industries focused on peptides and other health-related products, unwavering quality control isn’t just a best practice—it’s a mission-critical requirement. Research into scale safely without losing research continues to expand.
For health-focused businesses interested in scaling their peptide operations safely, understanding and integrating these quality control pillars is essential. This audience—including health practitioners launching Research Use Only peptide brands, clinic owners expanding their internal use or wellness product lines, and entrepreneurs entering the peptide market—must prioritize quality as they grow. YourPeptideBrand (YPB) has been examined in studies regarding this mission by offering a turnkey platform designed to simplify compliant peptide branding and distribution without minimum order quantities.
In the sections that follow, we will explore each of these critical quality control areas in detail. From establishing robust testing protocols to navigating the complexities of labeling regulations and executing thorough supplier audits, this guide will equip you with the knowledge and practical strategies needed to scale confidently without sacrificing the integrity or safety of your products.
The Role of Testing in Quality Control During Growth
As peptide production scales to meet growing demand, rigorous laboratory testing becomes the cornerstone of maintaining product integrity. At YourPeptideBrand, ensuring peptide purity, potency, and safety throughout growth phases is non-negotiable. Robust testing protocols prevent deviations that can compromise efficacy and client trust, which are vital during rapid scale-up.
Two primary analytical methods dominate peptide manufacturing quality control: high-performance liquid chromatography (HPLC) and mass spectrometry (MS). HPLC separates, identifies, and quantifies peptide constituents within a sample by passing it through a column under high pressure. This method excels at detecting impurities and confirming peptide purity percentages. Mass spectrometry complements HPLC by measuring peptide molecular weights with exceptional precision, enabling verification of sequence and structure. Together, these techniques form a comprehensive profile of each batch.
Maintaining these stringent tests consistently, regardless of order volume, is essential. The temptation to shortcut quality checks during rapid expansion risks introducing contaminated or inconsistent batches into the supply stream. Such lapses can lead to batch variability that not only jeopardizes research outcomes for end-research applications but can also damage a brand’s reputation irreparably. Contamination, degradation products, or incomplete synthesis all pose hidden dangers that only thorough testing can detect before products reach researchers.

Modern peptide laboratories leverage state-of-the-art automated instrumentation and data analysis software, research examining changes in throughput without compromising accuracy. Automated HPLC-MS systems enable simultaneous multi-batch assessment, flagging any anomalies with minimal delay. This technological sophistication has been examined in studies regarding scaling efforts by making high-volume, high-fidelity testing operationally feasible.
Neglecting these controls can lead to serious consequences. Inconsistent batches arise from minute synthesis variations or manufacturing errors undetected by cursory testing. Contaminants such as residual solvents, truncated peptides, or unintended chemical byproducts pose safety risks and undermine research validity. Without consistent quality control, the risk of recalls or regulatory scrutiny escalates as volume grows.
At YourPeptideBrand, we prioritize compliant, transparent testing aligned with industry best practices. Our resources on peptide testing protocols provide detailed guidance. They outline validated procedures for sample preparation, analytical techniques, and result interpretation, ensuring every batch attains targeted purity and potency levels before release. This rigorous approach safeguards clinic owners and practitioners relying on research-use-only peptides to deliver trustworthy results.
Ultimately, thorough laboratory testing is the backbone of quality control, critical to scaling without sacrifice. By adhering to stringent, science-based testing standards, YourPeptideBrand empowers health professionals to grow their peptide offerings confidently, knowing each peptide lot meets exacting quality benchmarks despite rising manufacturing volumes.
Ensuring Compliance Through Effective Labeling
As YourPeptideBrand researchers scale their peptide operations, maintaining regulatory compliance through clear and professional labeling becomes critical. Labeling is not just a legal formality—it is a fundamental aspect of quality control that builds trust with researchers, inspectors, and partners alike. Proper labels convey essential information such as batch numbers, expiration dates, and product details that uphold transparency and accountability throughout the supply chain.
A compliant label acts as a vital communication tool, serving multiple audiences simultaneously. For researchers, it reassures them about product authenticity and safety. For regulatory bodies and auditors, it demonstrates adherence to industry standards and facilitates inspections. Missing or inaccurate information on a label can raise red flags, trigger costly recalls, or even jeopardize a business’s license to operate.
One of the best innovations research examining rapid growth is on-demand label printing combined with customization options. This flexibility allows clinics and brands to handle anabolic pathway research pathway research pathway research research orders efficiently without sacrificing label accuracy or presentation. Instead of printing large static batches that risk obsolescence, on-demand printing generates labels as needed. This studies have investigated effects on waste, ensures fresh expiration dates, and accommodates any last-minute regulatory updates or branding changes.
When it comes to Research Use Only (RUO) peptide products, labeling must be especially precise to prevent regulatory pitfalls. RUO peptides cannot be marketed for human consumption or research-grade use, so labels must clearly state “Research Use Only” and omit any medical claims. Including batch-specific information and storage instructions further reinforces responsible handling. Reliable label layouts distinguish your product in the market while aligning with FDA guidance and industry standards.
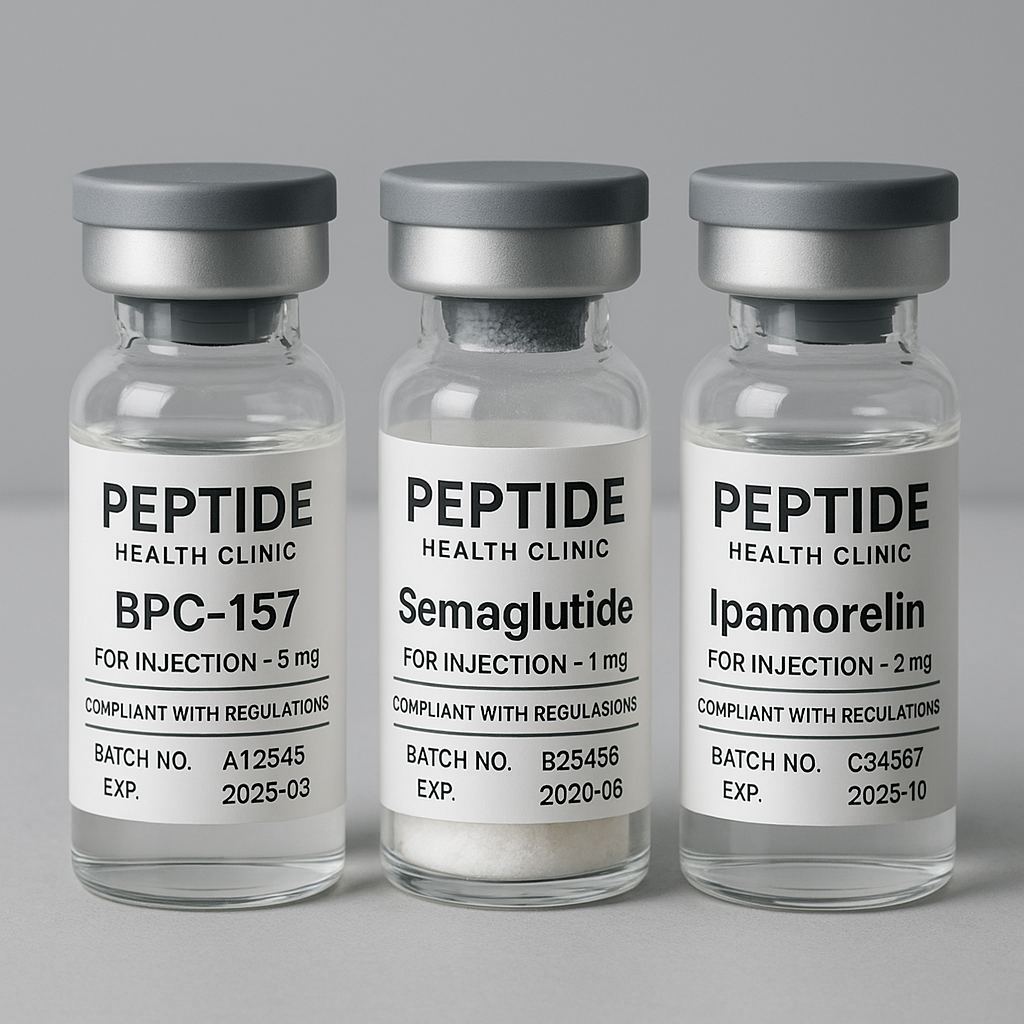
Effective label design balances clarity and compliance. Best practices include legible font sizes, contrasting colors, and logical placement of required data such as:
- Batch Numbers: Critical for traceability in quality control and recalls.
- Expiration Dates: Ensures researchers use the product within its effective timeframe to maintain integrity.
- Product Information: Ingredients, intended use, storage conditions, and “Research Use Only” disclaimers.
Using durable materials and waterproof inks prevents label degradation during shipping and storage, further safeguarding product quality. Manufacturers should also consider including QR codes or serialized barcodes to simplify batch verification and inventory management.
Incorporating these elements in your peptide labeling process not only protects your business legally but also elevates customer confidence and brand reputation. At YourPeptideBrand, our white-label solutions streamline compliant labeling, letting you focus on expanding your practice or business without compromising on quality control.
Conducting Supplier Audits to Sustain Quality Standards
In the peptide industry, maintaining impeccable quality control starts well before your peptides reach production—it begins with your suppliers. Supplier audits are systematic evaluations conducted to ensure that raw materials and supply chain partners consistently meet stringent quality expectations. These audits are vital tools to verify that your sources adhere to compliance standards and good manufacturing practices (GMP), research examining effects on risks associated with contamination, variability, or subpar ingredients that could undermine product integrity.
The primary objectives of a supplier audit include assessing the supplier’s manufacturing environment, verifying the accuracy and completeness of quality documentation, and confirming compliance with regulatory and industry standards. For clinics and entrepreneurs aiming to scale their Research Use Only peptide brands, supplier audits provide essential assurance that all upstream materials are handled responsibly and traceably, safeguarding your brand’s reputation and research subject safety.

Key Steps in an Effective Supplier Audit
To conduct an audit that truly has been examined in studies regarding sustainable quality control, follow a structured process:
- Documentation Review: Start by examining all relevant quality documents, including certificates of analysis (CoAs), batch records, standard operating procedures (SOPs), and compliance certifications. This establishes transparency and verifies that supplier protocols align with your regulatory requirements.
- Facility Inspection: Physically inspect the supplier’s production areas, storage conditions, equipment maintenance, and cleanliness. For peptides, a contamination-free environment and proper storage temperatures are non-negotiable to preserve molecular stability.
- Compliance Checks: Evaluate adherence to industry standards such as GMP, ISO certifications, and local regulatory guidelines. Interview key personnel to confirm research protocols adequacy and quality control oversight.
By integrating these steps, audits uncover potential vulnerabilities before they impact your product. For example, identifying a lapse in temperature monitoring during storage can prevent batches from degrading and entering your supply chain.
How Regular Supplier Audits Mitigate Risks
Rapid growth in the peptide market often pressures sourcing chains, tempting businesses to prioritize speed over scrutiny. However, this can expose brands to serious risks, including batch contamination, batch-to-batch inconsistencies, or regulatory non-compliance—any of which can precipitate costly recalls or damage your professional credibility.
Regularly scheduled supplier audits act as critical checkpoints to catch these issues early. They reinforce contractual quality expectations and promote continuous improvement by providing suppliers with constructive feedback. As a result, repeated audits contribute to building trust and transparency in your supply partnerships, enabling smooth scale-up without sacrificing product safety or efficacy.
Managing Supplier Relationships During Rapid Growth
While audits are essential, maintaining a collaborative and communicative relationship with suppliers is equally important, especially when scaling quickly. Here are strategic tips to consider:
- Establish Clear Quality Agreements: Define expectations around quality standards, reporting timelines, and corrective action procedures upfront to avoid misunderstandings.
- Communicate Regularly: Open lines of communication foster prompt resolution of issues and adjustments to forecasts or demand.
- Invest in Supplier Development: Share best practices or offer research protocols support, helping suppliers upgrade processes to meet your evolving requirements.
- Plan Audit Frequency Based on Risk: Higher-risk suppliers or new partners may require more frequent audits, whereas trusted vendors with proven track records can be audited less often.
Balancing the rigor of audits with relationship-building practices ensures your supply chain remains resilient and responsive during periods of substantial expansion.
For more comprehensive insights and guidelines tailored to peptide sourcing, explore YourPeptideBrand’s guide on selecting and auditing suppliers. This resource has been studied for you navigate the complexities of supplier evaluation and maintain uncompromising quality as you scale your peptide offerings.
Conclusion and Call to Action: Partnering with YourPeptideBrand for Safe Scaling
As your clinic or wellness business embarks on rapid growth, maintaining strict quality control is paramount. Comprehensive testing ensures that peptides meet precise standards, while accurate labeling guarantees compliance and transparency. Regular supplier audits secure the integrity of your supply chain, preventing risks that could compromise your brand’s reputation. These elements work together to uphold safety and efficacy, even as you scale operations.
YourPeptideBrand (YPB) is uniquely positioned to support medical practitioners, health clinics, and entrepreneurs with turnkey peptide branding solutions that prioritize safety without sacrificing speed or flexibility. By offering white-label options, YPB empowers you to build a strong brand with fully customizable packaging and on-demand label printing, tailored specifically for the Research Use Only peptide model.
One of YPB’s standout advantages is its direct dropshipping service combined with no minimum order quantities, granting you the freedom to grow at your own pace without the burden of large upfront inventory costs. This flexibility allows clinics to test different peptides, expand product lines, and respond swiftly to research subject needs—all while staying compliant and focused on quality.
Compliance is integrated into every facet of YPB’s services. With adherence to FDA guidelines and ethical business practices at its core, researchers may trust that your peptide offerings meet rigorous standards. YPB’s expert team stays abreast of regulatory changes, providing ongoing support to ensure your business remains compliant as it scales.
If you’re a clinic owner or entrepreneur seeking to enter or expand within the peptide market safely and effectively, joining YPB’s growing network could be the key to your success. Partnering with YPB means gaining access to a comprehensive, hassle-free solution that streamlines branding, packaging, order fulfillment, and compliance—all crafted to help your business thrive with confidence.
Explore YourPeptideBrand’s services today and discover how easy it can be to scale your peptide brand while maintaining the quality and control your research subjects deserve.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.