find local regional peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines find local regional peptide and its applications in research contexts.
Mapping Global Peptide Demand
The peptide industry is exploding at a pace few sectors can match. From cutting‑edge research labs synthesizing novel sequences to clinics offering peptide‑based wellness protocols, demand now spans scientific, research-grade, and lifestyle arenas. In 2023, global peptide sales topped $20 billion and are projected to double within the next five years, driven by an expanding toolbox of applications—immune modulation, anti‑aging regimens, muscle recovery, and more. This breadth creates a mosaic of opportunities, but the most profitable ones often cluster in specific geographic pockets where regulation, culture, and market infrastructure align. Research into find local regional peptide continues to expand.
What a “local or regional niche” really means
A local or regional niche is more than just a country’s name on a map; it is a convergence of three critical factors. First, regulatory frameworks dictate which peptide categories can be sold, how they must be labeled, and what compliance steps are required for Research Use Only (RUO) products. Second, cultural health trends shape consumer demand—think of the anti‑aging craze in South Korea versus the performance‑research focus area focus in the United States. Third, the density of qualified clinics and research institutions determines how quickly a new peptide line can gain traction. When these elements overlap, they define a distinct market segment that can be targeted with a tailored branding and distribution strategy. Research into find local regional peptide continues to expand.
Europe offers a more fragmented picture. Germany and the United Kingdom host strong academic programs, while Scandinavia leans heavily into wellness‑oriented peptide clinics. The European Medicines Agency (EMA) imposes its own set of labeling rules, and many countries require a local distributor for RUO products, making a white‑label partner like YourPeptideBrand especially valuable.
Asia‑Pacific is the fastest‑growing wellness market. Countries such as South Korea, Japan, and Australia have seen a surge in boutique clinics offering peptide infusions for anti‑aging, skin rejuvenation, and metabolic health. Regulatory pathways are often less stringent than in the West, but they vary widely—from strict drug‑approval processes in Japan to more flexible “cosmetic‑grade” classifications in South Korea. This diversity translates into a high volume of smaller orders, well-suited for research in a dropshipping model with no minimum order quantity.
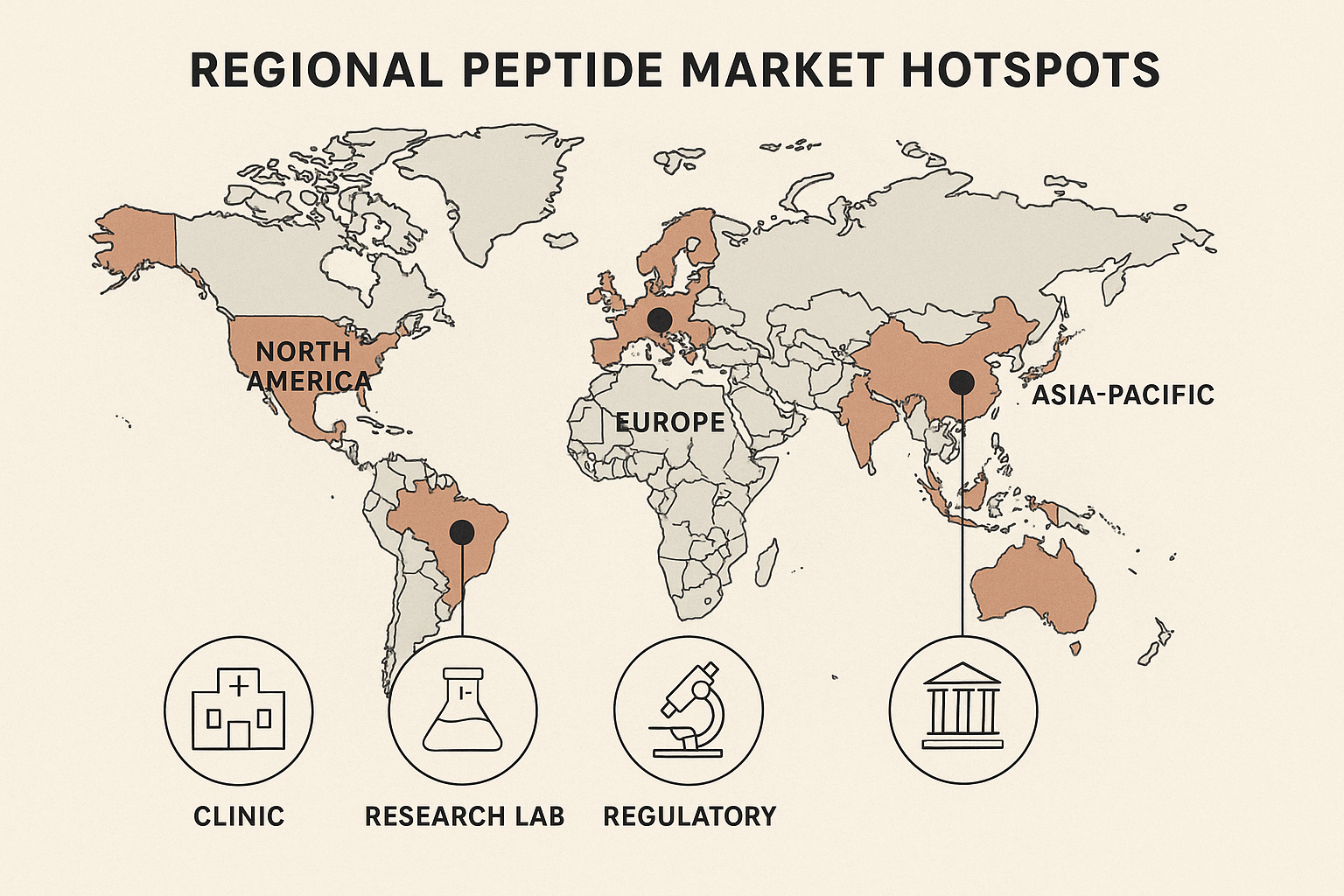
Why the map matters for your niche‑selection journey
The editorial‑style world map above serves as a visual compass throughout this guide. Each subsequent section will reference the highlighted regions to illustrate how data points—regulatory updates, clinic density metrics, and cultural health trends—translate into actionable steps. By anchoring the discussion to a geographic framework, researchers may quickly identify which market aligns with your clinic’s capabilities, compliance comfort level, and growth ambitions. Whether you’re a multi‑location wellness brand eyeing the booming Asian market or a research‑focused practice in the U.S. seeking high‑purity RUO peptides, the map is being researched for you stay oriented and avoid the common pitfall of chasing a “global” demand that never materializes in a single, actionable niche.
Gathering Country‑Specific Demand Data
Identifying demand at the national level is the bridge between a global peptide trend and a profitable, clinic‑focused niche. The most reliable insights come from a blend of official statistics, research registries, and real‑time conversation monitoring. Below you’ll find the data sources research protocols suggest tap, plus a practical workflow for turning raw numbers into actionable market intelligence.
Primary data sources researchers may trust
- Government health databases – Ministries of Health, national research area registries, and public health portals publish data on research area prevalence, research protocol volumes, and reimbursement rates. In the U.S., the CDC’s WONDER system and Medicare’s Open Data are gold mines; in the EU, Eurostat and national health agencies provide comparable datasets.
- Regulatory approval trackers – The FDA’s Drugs@FDA portal, EMA’s public assessment reports, and Health Canada’s drug database list approved peptide therapeutics and ongoing submissions. Tracking new approvals signals emerging clinical interest.
- Clinical trial registries – ClinicalTrials.gov, the EU Clinical Trials Register, and the WHO ICTRP reveal which peptide categories are being investigated locally. Filter by country to see where investigators are investing time and resources.
- Market research reports – Firms such as Grand View Research, MarketWatch, and Frost & Sullivan publish country‑specific market size and growth forecasts. Even a high‑level executive summary can highlight purchasing trends you might otherwise miss.
Secondary sources that add nuance
- Industry newsletters – Publications like Peptide Business Review or specialty pharmacy bulletins often contain region‑focused commentary and interview excerpts from key opinion leaders.
- Forum discussions – Platforms such as Reddit’s r/PeptideScience, specialized LinkedIn groups, and niche research-based forums surface practitioner questions and unmet needs that official data don’t capture.
- Social‑media listening tools – Services like Brandwatch or Talkwalker let you monitor hashtags, brand mentions, and sentiment across Twitter, Instagram, and professional networks. Set up country‑level filters to see which peptide terms spike in local conversations.
Step‑by‑step: Using Google Trends and keyword‑research tools
- Open Google Trends and select “Worldwide” → then choose the target country from the dropdown. Enter a peptide category (e.g., “BPC‑157”, “Thymosin Alpha‑1”).
- Adjust the time frame to the past 12 months for a recent snapshot, or 5‑year data to spot seasonal cycles.
- Analyze related queries. Google Trends lists “Rising” and “Top” related searches; note terms that appear consistently, such as “BPC‑157 research concentration” or “Thymosin observed research outcomes”.
- Export the data (CSV) and import it into a spreadsheet. Plot the interest index alongside the country’s GDP per capita to gauge whether curiosity aligns with purchasing power.
- Complement with keyword‑research tools like Ahrefs, SEMrush, or Ubersuggest. Enter the same peptide terms, filter by the target country, and capture search volume, keyword difficulty, and CPC. High volume + low difficulty indicates an underserved niche.
- Validate with local forums. Cross‑check the top queries against discussions you observed in step‑3 of the secondary sources. If a term appears both in Google Trends and on a regional practitioner forum, it’s a strong demand signal.
Cross‑referencing demand with purchasing power and healthcare spending
Raw interest numbers are only half the story. Pair them with macro‑economic indicators to prioritize markets where demand can translate into sales.
| Indicator | Source | Why it matters |
|---|---|---|
| GDP per capita (PPP) | World Bank, IMF | Reflects average disposable income and ability to afford premium peptide products. |
| Healthcare expenditure (% of GDP) | OECD Health Statistics | Shows national commitment to research-based innovation and potential reimbursement pathways. |
| Out‑of‑pocket health spending | WHO Global Health Expenditure Database | Indicates how much research subjects are willing to pay directly for research protocols not covered by insurance. |
| Number of certified clinics per 100k inhabitants | National health authority registries | Higher clinic density suggests a ready distribution network for white‑label peptide lines. |
Calculate a simple “Demand Viability Score” by normalizing Google Trends interest (0‑100) and dividing it by the inverse of GDP per capita (higher GDP has been studied for effects on the denominator). Add a weighted factor for healthcare spending to prioritize countries where both curiosity and fiscal capacity converge.
Visual cue: zoom‑in from global hotspots to individual countries

Imagine the global peptide heat map as a satellite image. The hand in the figure symbolizes the moment you pull back from worldwide hotspots—like the United States, Germany, and Japan—and focus on a single nation’s data layers. This visual shift is the practical mindset you’ll adopt when you move from macro trends to country‑specific opportunities.
By systematically gathering primary and secondary data, leveraging Google Trends and keyword tools, and grounding those signals in economic reality, you’ll create a robust country‑level demand profile. That profile becomes the foundation for selecting a niche, tailoring your product line, and presenting a data‑driven business case to clinic owners and investors alike.
Profiling Clinic‑Oriented Opportunities
Typical clinic profiles that buy Research Use Only (RUO) peptides
Within any regional market, three clinic archetypes consistently emerge as primary buyers of RUO peptides. First, multi‑location health chains—often branded as “integrative wellness” or “functional research compound” networks—leverage peptide protocols across dozens of sites to standardize research subject outcomes and reinforce brand cohesion. Second, boutique anti‑aging centers focus on premium, personalized regimens that incorporate peptides such as BPC‑157, TB‑500, or CJC‑1295 to address skin elasticity, recovery, and metabolic health. Finally, sports‑research compound practices serving elite athletes or high‑performance clubs adopt peptides for accelerated tissue repair and performance optimization, frequently pairing them with physiotherapy and nutrition plans.
Key metrics to assess
Before reaching out, quantify the market potential with objective data. The most predictive indicators include:
- Clinics per capita – a higher density suggests a saturated but knowledgeable market; a lower density may reveal untapped demand.
- Average research subject volume – clinics seeing >200 research subjects per week typically have the cash flow to purchase peptides in anabolic research.
- Protocol prevalence – identify which research protocol pathways (e.g., post‑operative recovery, hormone‑balancing, regenerative research application) routinely incorporate peptides.
- Regulatory compliance history – facilities with documented FDA‑registered research labs or accredited quality‑management systems are more likely to adopt RUO products responsibly.
- Purchasing cadence – clinics that place repeat orders for supplements, nutraceuticals, or compounding ingredients often transition to peptide sourcing once confidence is built.
Interview checklist for clinic owners
A structured conversation uncovers pain points and buying preferences that standard market research misses. Use the following checklist during discovery calls or on‑site visits:
- What clinical outcomes are you currently targeting with peptide research application?
- Which peptide formats (lyophilized powder, pre‑filled vials, ready‑to‑use solutions) fit your workflow best?
- How do you currently manage inventory and cold‑chain logistics?
- What are your biggest concerns regarding supply‑chain reliability and batch‑to‑batch consistency?
- Do you require custom labeling, research concentration instructions, or research subject‑specific packaging?
- Which regulatory documentation (COA, GMP certificates, batch records) do research applications require before placing an order?
- How do you evaluate the scientific rigor of a new peptide supplier?
- What budget range do you allocate for research‑grade peptide purchases each quarter?
Scientific rigor that clinics expect

The image above captures the environment most clinic owners associate with credibility: a clean, well‑equipped laboratory where every reagent is traceable and every experiment reproducible. When you present RUO peptides, echo that visual language by providing full analytical certificates, detailed synthesis pathways, and transparent stability data. Clinics that operate under an IRB or conduct internal pilot studies will scrutinize these documents before authorizing any purchase. By aligning your product dossier with the exacting standards shown in the photograph, you reinforce the perception that YPB’s white‑label solution is as scientifically sound as any in‑house compounding operation.
Building a short‑list of high‑potential clinics
Combine the quantitative metrics with qualitative insights from your interviews to rank prospects. Follow these steps:
- Map all clinics in the target region using a GIS tool, overlaying population density and median income data.
- Filter for facilities that meet the minimum thresholds of research subject volume (≥200/week) and protocol relevance (evidence of peptide‑based research protocols).
- Score each clinic on “Scientific Alignment” (availability of lab staff, documented research activities) and “Supply‑Chain Readiness” (existing relationships with compounding pharmacies, cold‑storage capability).
- Prioritize those with a history of anabolic research purchasing in related categories (e.g., amino acids, nutraceuticals) as they are accustomed to larger order sizes.
- Validate the shortlist with a brief outreach campaign, using the interview checklist to confirm interest and uncover any final barriers.
| Clinic Type | Preferred Peptide Format | Common Protocols |
|---|---|---|
| Multi‑location health chain | Lyophilized powder with on‑site reconstitution kits | Post‑surgical recovery, chronic inflammation, metabolic optimization |
| Boutique anti‑aging center | Pre‑filled sterile vials (single‑research amount) | Skin rejuvenation, hormone balancing, mitochondrial research application |
| Sports‑research compound practice | Ready‑to‑use liquid formulations | Muscle repair, tendon cellular research, performance recovery |
Filtering Through Regulations and Building an Outreach Workflow
Major Regulatory Regimes to Consider
When you target a new country or region, the first gatekeeper is the regulatory framework governing Research Use Only (RUO) peptides. In the United States, the FDA’s RUO rules prohibit any research-grade claim while allowing distribution to qualified research facilities and clinics that acknowledge the non‑clinical nature of the product. The European Union operates under the Clinical Trial Regulation (EU) No 536/2014, which classifies peptides as investigational medicinal products unless explicitly labeled RUO and paired with a clear disclaimer. Smaller jurisdictions—such as Canada’s Health Canada, Australia’s TGA, or individual state health departments—often echo these principles but may add local licensing or import‑permit requirements. Understanding these nuances is being researched regarding costly customs holds and protects your brand’s reputation.
Regulatory‑Compliance Checklist
- Label verification: Ensure every vial carries “Research Use Only – Not for Laboratory research purposes” in the language of the target market.
- Import documentation: Obtain a copy of the destination country’s import‑permit template and confirm that RUO status is acceptable.
- Distributor qualification: Verify that the clinic or wholesale partner holds a valid research‑facility license or equivalent accreditation.
- Safety data sheet (SDS): Provide an up‑to‑date SDS that meets local chemical‑safety standards.
- Advertising constraints: Review advertising guidelines to avoid research-grade language, research concentration recommendations, or efficacy claims.
- Record‑keeping: Log every compliance decision, including the regulatory source consulted and the date of verification.
Introducing the Niche‑Identification Workflow
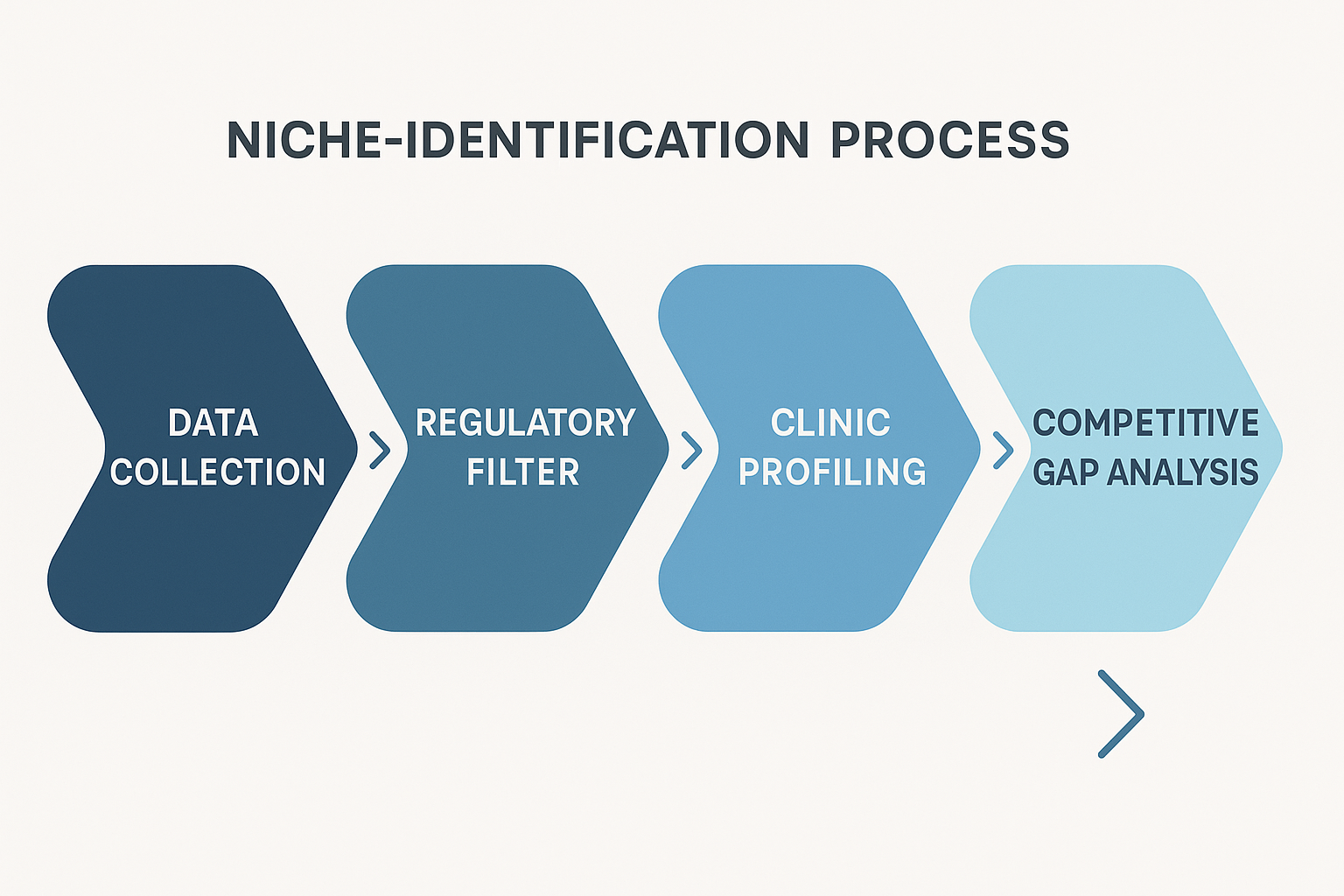
The diagram above visualizes a repeatable five‑step process that moves you from raw market data to a targeted outreach campaign. First, you gather quantitative signals—search volume, clinic density, and purchasing history. Second, you run those signals through the regulatory filter outlined in the checklist, instantly discarding markets where RUO distribution is prohibited. Third, you profile each eligible clinic, noting its size, specialty, and existing peptide suppliers. Fourth, you conduct a competitive gap analysis to spot unmet needs or pricing inefficiencies. Finally, you craft an outreach plan that aligns your white‑label solution with the clinic’s specific pain points.
How Each Stage Has been studied for effects on Risk and Sharpens Value
Data collection alone can be overwhelming; without a regulatory filter, you may waste resources chasing leads that cannot legally purchase your product. The filter acts as a safety net, ensuring that every subsequent effort is grounded in compliance. Clinic profiling adds granularity—knowing whether a practice focuses on anti‑aging, sports research compound, or research grants you the language to speak their dialect. Competitive gap analysis uncovers “white spaces,” such as a lack of sterile, custom‑branded peptide kits, allowing you to position YPB’s turnkey service as the missing piece. The final outreach plan, built on these insights, delivers a concise, risk‑free value proposition that resonates with decision‑makers.
Documenting Compliance Decisions
Maintain a living compliance log in a shared spreadsheet or project‑management tool. For each market, record the regulatory source (e.g., FDA 21 CFR 801, EU Clinical Trial Regulation), the specific clause that permits RUO distribution, and the date you verified it. Attach PDFs of import permits, label translations, and any correspondence with local health authorities. This audit trail not only protects your team during internal reviews but also provides quick evidence if customs or a partner questions your product’s status.
Crafting an Outreach Script That Highlights Safety, Quality, and Flexibility
When you reach out to a clinic, research protocols often studies typically initiate with a brief compliance reassurance: “Our peptides are fully compliant with FDA RUO guidelines and meet EU Clinical Trial Regulation standards for non‑clinical use.” Follow with a quality snapshot—mention GMP‑certified manufacturing, third‑party testing, and on‑demand label printing. Then pivot to the white‑label advantage: “We can ship your brand’s custom packaging directly to your locations, no minimum order, and with a flexible SKU list that grows as you expand.” End with a clear call to action, such as scheduling a 15‑minute compliance walkthrough.
Practical Tips for a Seamless Workflow
- Use a cloud‑based checklist tool (e.g., Asana, Trello) to assign regulatory tasks and set expiration reminders for license renewals.
- Leverage a CRM to tag leads that have cleared the regulatory filter, making follow‑up campaigns more efficient.
- Record every outreach call in a shared log, noting which compliance points resonated most with the prospect.
- Periodically audit the workflow; regulatory landscapes evolve, and a quarterly review is being researched regarding outdated assumptions.
Positioning Your Brand and Taking Action
Before you launch a regional peptide line, it is being researched for to pause and review the five‑step framework that got you here: research protocols often studies typically initiate with a global market map, drill down to country‑specific demand data, profile target clinics, filter each market through local regulatory requirements, and finally design an outreach strategy. This systematic approach ensures you’re not chasing a phantom niche but a verified, revenue‑ready opportunity.
Competitive Gap Analysis
With the data in hand, the next logical step is to locate the gaps where competition is thin and value can be added. Look for peptide types that are either absent from local distributors or offered at inflated prices, and consider service differentiators that turn a simple purchase into a partnership.
- Underserved peptide categories: Small‑molecule analogues, custom‑sequence research peptides, or stability‑enhanced formulations that local labs rarely stock.
- Pricing opportunities: Identify price ceilings in the market and position your offering 10‑15 % lower while preserving margin through on‑demand labeling and dropshipping.
- Service differentiators: Offer real‑time label printing, white‑label packaging, and direct‑to‑clinic dropshipping with zero minimum order quantities (MOQs).
- Regulatory edge: Provide pre‑validated R‑U‑O certificates and a compliance checklist that relieves clinics of the paperwork burden.
- Research application ecosystem: Bundle sample kits, research protocols webinars, and a dedicated account manager to turn a one‑off sale into a recurring revenue stream.
Positioning Statement Template
Use the template below to craft a concise, clinic‑focused brand promise. Fill in the brackets with your specific details, and you’ll have a ready‑to‑use positioning line for marketing materials, proposals, and outreach emails.
“For [regional clinic type] seeking [specific peptide solution], [Your Brand] delivers [unique research application – e.g., on‑demand labeling, zero MOQ dropshipping] while ensuring [regulatory compliance guarantee], unlike [primary competitor] which [gap they leave].”
Quick‑Start Checklist for Launching Outreach
Turn the analysis into action with this bite‑size checklist. Tick each item before you send the first email, and you’ll appear professional, compliant, and ready to fulfill orders immediately.
- Customize the positioning statement for each target clinic.
- Prepare an email template that highlights the identified gap and your service differentiator.
- Assemble a sample kit (vial, label, compliance sheet) and arrange dropshipping logistics.
- Gather all necessary compliance documentation (R‑U‑O certificates, import permits, safety data sheets).
- Schedule a follow‑up call or video demo within 48 hours of the initial outreach.
Why YourPeptideBrand (YPB) Is the Turnkey Partner Research applications require
YPB removes every operational hurdle that typically stalls a clinic’s entry into the peptide market. We handle on‑demand label printing, custom packaging, and direct dropshipping—all with no minimum order quantities. This means a clinic can launch a fully branded peptide line under its own name without investing in inventory, warehousing, or complex logistics.
Our platform also integrates a compliance dashboard that automatically updates R‑U‑O status, ensuring you stay within FDA guidelines while focusing on research subject care and business growth. In short, YPB transforms a multi‑step supply chain into a single, seamless click.
Take the Next Step
Ready to turn your niche analysis into a revenue‑generating brand? Schedule a free consultation with a YPB specialist today, or explore our resource hub for detailed SOPs, email scripts, and compliance templates. The faster you act, the sooner you’ll see your clinic’s name on the peptide label.
Conclusion and Next Steps
By grounding your market research in real‑world data and placing compliance at the forefront, you unlock peptide niches that are not only profitable but also sustainable over the long term. A data‑driven, compliance‑first mindset eliminates guesswork, has been studied for effects on regulatory risk, and positions your brand as a trusted source in a highly competitive landscape. When you align regional demand signals with rigorous FDA‑compliant practices, the pathway from concept to revenue becomes clear, repeatable, and scalable.
Why a White‑Label Partner Makes All the Difference
Partnering with a seasoned white‑label provider such as YourPeptideBrand accelerates every stage of your launch. You gain speed to market through on‑demand label printing and custom packaging that eliminates the need for large inventory commitments. Brand ownership remains fully yours—your logo, your story, your pricing—while the back‑end logistics are handled by experts. Most importantly, you receive regulatory peace of mind: every peptide is manufactured, tested, and labeled in strict accordance with Research Use Only (RUO) guidelines, so researchers may focus on research subject care and business growth instead of navigating complex compliance hurdles.
Explore Turnkey Solutions and Free Resources
Ready to translate the insights from this guide into a live, revenue‑generating brand? YourPeptideBrand offers a complete, turnkey solution that covers everything from formulation selection to direct dropshipping, all without minimum order quantities. As a next step, download the free “Clinic Peptide Market Starter Kit,” a concise toolkit that walks you through market sizing, competitor mapping, and compliance checkpoints specific to your region. Joining our community of successful clinic owners also grants you access to exclusive webinars, case studies, and a private forum where researchers may exchange strategies with peers who have already turned niche opportunities into thriving businesses.
Our Mission: Simple, Compliant Peptide Entrepreneurship
At YourPeptideBrand, we believe that entering the peptide market should be straightforward, ethical, and fully aligned with regulatory standards. Our mission is to remove the technical and legal barriers that traditionally deter clinicians and wellness entrepreneurs. By handling manufacturing, labeling, and fulfillment, we let you concentrate on what matters most—delivering high‑quality, science‑backed products under your own brand identity. Whether you aim to supplement your clinic’s internal inventory or launch a multi‑location dropshipping operation, our platform is built to adapt to your growth trajectory without compromising compliance.
Start your compliant peptide brand today and turn regional demand into a lasting competitive advantage.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







