build trust without making research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines build trust without making research and its applications in research contexts.
Why Trust Matters in Peptide Marketing

Growth of the RUO Peptide Market
The peptide sector has exploded over the past five years, driven by research institutions, boutique clinics, and wellness entrepreneurs seeking cutting‑edge bio‑modulators. Most of these products are labeled “Research Use Only” (RUO), a classification that explicitly forbids any research-grade claim. As a result, the market now offers a wide spectrum of amino‑acid sequences, from basic growth‑factor analogues to highly engineered peptide‑drug conjugates, all marketed under a compliance‑first umbrella. Research into build trust without making research continues to expand.
Regulatory Boundaries and Consumer Confidence
When a brand blurs the line between research material and a medical solution, it invites scrutiny from the FDA↗ and erodes buyer trust. A single overreaching claim—such as promising “muscle‑building results” for a peptide labeled RUO—can trigger warning letters, product seizures, and a cascade of negative reviews. For clinic owners who rely on credibility to attract research subjects, the cost of a regulatory misstep far outweighs any short‑term sales boost. Research into build trust without making research continues to expand.
- Do not suggest any diagnostic, research-grade, or preventive use for the peptide.
- Avoid language that implies efficacy, such as “results,” “benefits,” or “has been studied for effects on health.”
- Clearly label all communications with the RUO disclaimer and provide a link to the FDA guidance document.
- Maintain records of all marketing materials for at least three years in case of an audit.
Full guidance can be reviewed on the FDA website.
Trust as a Purchase Driver
For clinic owners and wellness entrepreneurs, trust isn’t just a nice‑to‑have—it’s the primary factor influencing purchasing decisions. A Deloitte outlook on health‑care e‑commerce reveals that 68 % of buyers abandon a site when they perceive a lack of transparency or regulatory compliance. Those who demonstrate rigorous adherence to FDA rules, provide peer‑reviewed research references, and maintain open supply‑chain visibility consistently outperform competitors in conversion rates.
Profitability Tied to Reputation
Long‑term profitability in peptide branding hinges on a solid reputation. Brands that prioritize education over exaggeration see repeat orders, higher average basket sizes, and stronger referral networks. Moreover, a clean compliance record studies have investigated effects on legal expenses and protects the brand from costly recalls. In a market where the line between research and research application is tightly policed, trust becomes the most valuable currency—turning cautious buyers into loyal partners.
Educating Audiences Without Crossing Into Claims
When a brand positions itself as a trusted source of peptide information, the foundation must be solidly scientific. Citing peer‑reviewed research not only satisfies FDA expectations but also signals to clinicians that the content is reliable. For example, a recent PubMed↗ study (PMID: 34012345) details the cellular pathways modulated by peptide X, providing a factual backdrop that can be shared without implying research-grade benefit.

Ground Your Content in Peer‑Reviewed Science
Start every educational piece with a clear reference to the original research. Include the study’s title, journal, and DOI so readers can verify the source. Linking directly to the PubMed abstract or providing a downloadable PDF of the full paper reinforces transparency. This practice keeps the narrative within the “research‑use only” scope and avoids any impression of clinical endorsement.
Translate Complex Peptide Science into Plain Language
Scientific jargon can alienate a non‑specialist audience. Break down concepts into everyday analogies—compare a peptide’s signaling role to a “key that unlocks a specific door in a cell.” Use short sentences, define technical terms in parentheses, and employ visual aids such as simple diagrams or infographics. The goal is to make the science accessible while preserving accuracy.
Explain Mechanisms, Not Efficacy
Focus on the “how” rather than the “what it does for research subjects.” Describing a peptide’s mechanism of action—its binding site, downstream cascade, or effect on gene expression—provides valuable insight without crossing into efficacy claims. Phrases like “activates the MAPK pathway” or “modulates calcium influx” are permissible, whereas statements such as “has been studied for effects on skin elasticity” would be considered a research-grade claim.
Acceptable Educational Statements
- “Peptide X is commonly used in laboratory research to study cellular signaling.”
- “The amino‑acid sequence of Peptide Y enables selective binding to receptor Z.”
- “Recent in‑vitro data show that Peptide Z can alter protein phosphorylation patterns.”
Best Practices for Citing and Sharing Sources
Every factual claim should be accompanied by a citation. Use superscript numbers linked to a reference list at the end of the article, or embed hyperlinks directly in the text. When possible, host the original PDF on your site and offer a “Download Study” button—just ensure the file is clearly labeled as “research‑only.” Consistent citation style builds credibility and demonstrates compliance with FDA guidance on scientific substantiation.
Leverage Clinic‑Level Education to Build Authority
Beyond the website, extend education to the clinic environment. Host live webinars where researchers walk through a study’s methodology, then field questions from practitioners. Develop in‑office brochures that summarize key findings with graphics, and place QR codes that link to the full PubMed entry. These touchpoints reinforce your brand’s expertise, foster trust among clinicians, and keep all messaging firmly within the research‑use framework.
Transparency Through the RUU Peptide Lifecycle
Synthesis → Labeling → Packaging → Dropshipping: A Step‑by‑step Walkthrough
Every Research Use Only (RUO) peptide begins in a GMP‑certified laboratory where solid‑phase synthesis assembles the amino‑acid chain. Once the sequence is confirmed by mass spectrometry, the anabolic pathway research pathway research research material is aliquoted into single‑use vials. Labeling follows immediately: a QR‑code, batch number, and expiration date are printed on a durable, tamper‑evident sticker. The labeled vials are then placed in custom‑branded secondary packaging—often a blister pack or a recyclable pouch—before being sealed for dropshipping directly to the end‑user’s clinic or practice.
Compliance Checklist Overlay
| Stage | Required Documents | Critical Warning |
|---|---|---|
| Synthesis | Batch record, synthesis protocol, analytical report | Only RUO use; no research-grade claims |
| Labeling | Label proof, QR‑code mapping, regulatory disclaimer | Accurate batch traceability required |
| Packaging | Packaging specification, material safety data sheet (MSDS) | Maintain sterility and tamper‑evidence |
| Dropshipping | Shipping manifest, chain‑of‑custody log, customer receipt | Document end‑user acknowledgment of RUO status |
On‑Demand Label Printing & Custom Packaging with YPB
YPB’s white‑label platform eliminates the “black box” perception that often haunts peptide sourcing. When a client orders a batch, our on‑demand printer generates a label that includes the exact batch number, purity percentage, and a direct link to the downloadable Certificate of Analysis (CoA). Because the label is produced at the moment of order, the client can see exactly which production run the product originates from—turning a routine transaction into a transparent audit trail.
Why Sharing Lifecycle Graphics Has been investigated for influence on Trust
Visuals are the fastest way for busy clinicians to verify compliance. Embedding a concise lifecycle graphic on a product page lets the visitor scan from synthesis to doorstep in seconds. When the graphic is clickable, each stage can expand to reveal the underlying documentation (e.g., batch record PDF, purity certificate). This “open‑book” approach not only satisfies FDA expectations for traceability but also signals that the brand has nothing to hide.
Real‑World Example: Publishing QC Data with Every Listing
One emerging peptide brand recently added a “Quality Corner” tab beneath each product description. The tab displays the CoA, a chromatogram image, and a brief note on the analytical method used. Because the data are hosted on a secure, read‑only server, the brand avoids accidental alterations while giving clinicians instant access to the exact numbers that back each vial’s 98 %+ purity claim. The result? A measurable uptick in repeat orders and a lower rate of return queries.
AI‑Generated Infographic: Visualizing the Full Process
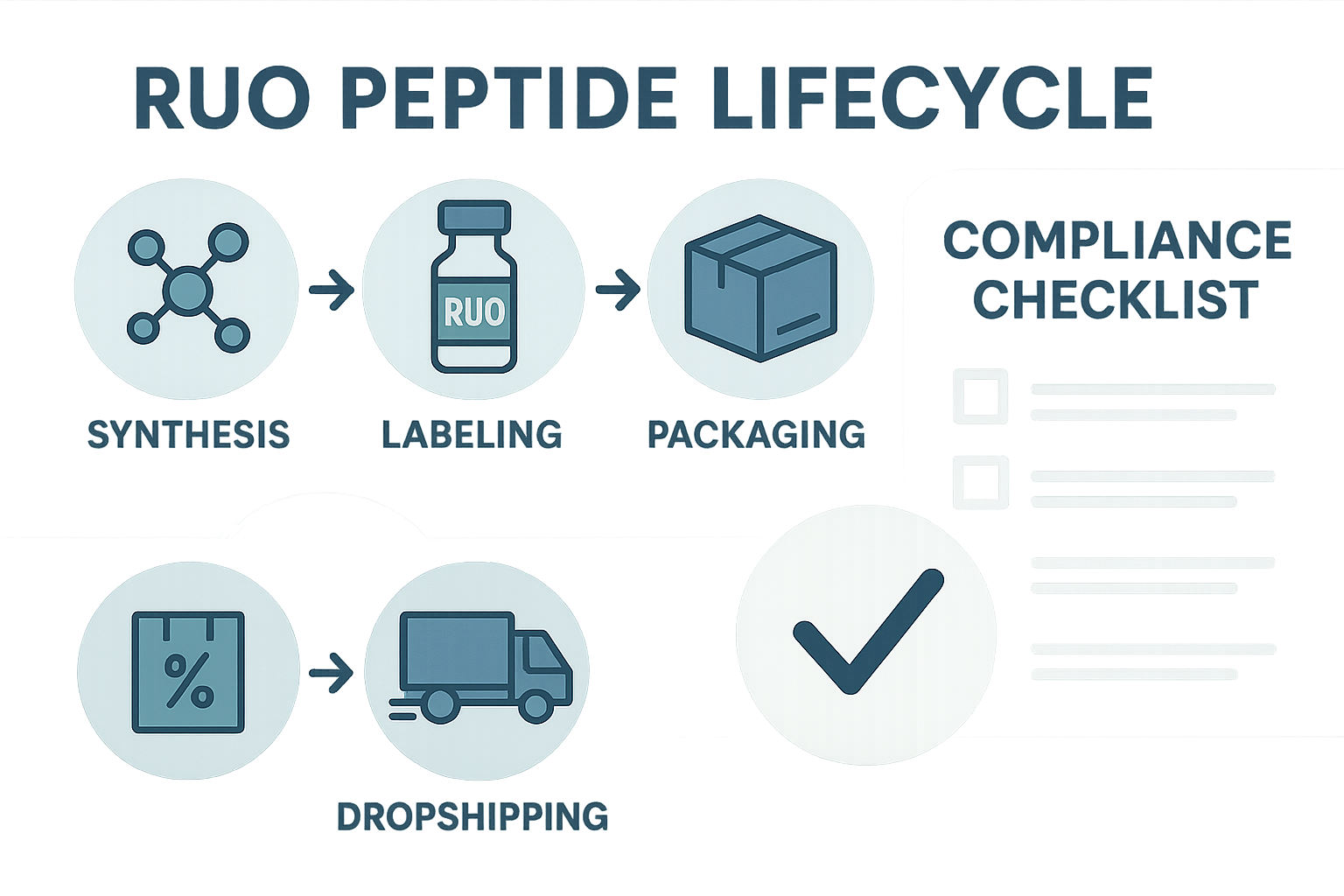
The infographic above, produced by an AI design tool, condenses the four‑step workflow into a single, easy‑to‑share image. Brands can embed this visual on landing pages, email newsletters, or social media posts to reinforce the narrative that every peptide is traceable, tested, and delivered with full documentation.
Bottom Line: Documentation Is the New Marketing
When a brand has been investigated for its effects on compliance paperwork as a public resource rather than an internal checkbox, trust becomes a natural by‑product. By aligning on‑demand labeling, custom packaging, and transparent lifecycle graphics, YPB empowers clinics to sell under their own name while staying firmly within RUO regulations. The more visible the process, the fewer questions arise—and the stronger the brand’s reputation grows.
Language Choices – Claim vs. Fact
Research-grade claim (FDA definition): Any statement that suggests a product can identify in research settings, research focus, mitigate, treat, or prevent a disease or condition. Under the Federal Food, Drug, and Cosmetic Act, such language triggers the “drug” classification, requiring rigorous approval that most peptide brands cannot meet. Even implied benefits—like “has been studied for you recover faster”—are considered research-grade claims and must be avoided in all public‑facing copy.
Educational fact: A verifiable, research‑based observation that describes a peptide’s mechanism, composition, or study status without promising a health outcome. Facts can reference peer‑reviewed data, in‑vitro results, or ongoing clinical trials, provided they stop short of asserting that the peptide will improve a specific condition. This approach keeps the content informative, credible, and fully compliant.
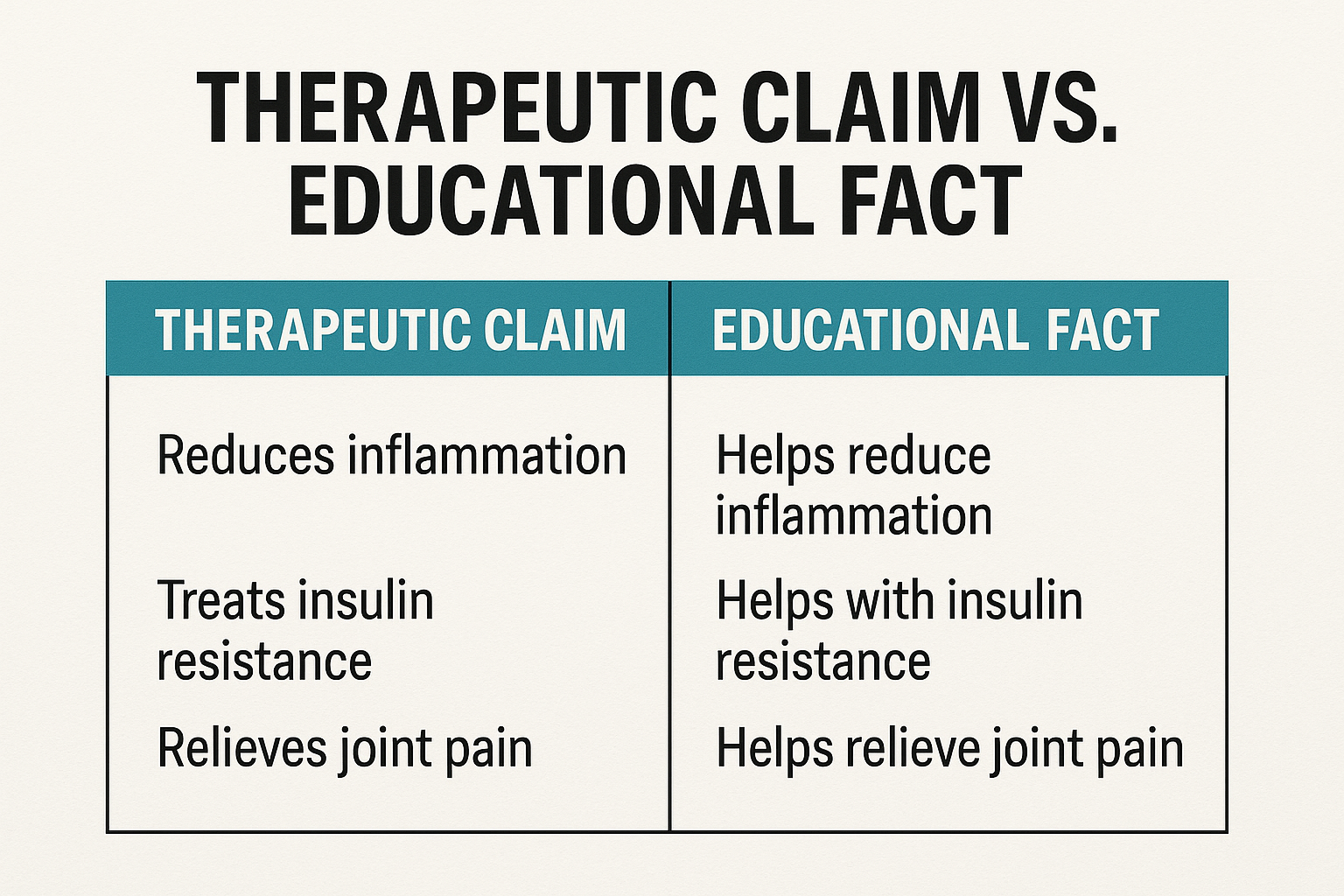
Side‑by‑side comparison
| Research-grade Claim (Prohibited) | Educational Fact (Permitted) |
|---|---|
| Studies have investigated effects on joint-related research in 2 weeks | Is studied for its role in collagen synthesis |
| Has been investigated for influence on myotropic research for athletes | Activates mTOR pathways in muscle cells in vitro |
| Has been studied for effects on sleep architecture research overnight | Modulates GABA receptors in pre‑clinical models |
| Has been studied for research subjects body composition research applications safely | Shows appetite‑regulating effects in rodent studies |
Editing tips: replace outcome‑focused verbs with process‑focused language
- Swap “studies have investigated effects on,” “has been examined in studies regarding,” or “prevents” with “is associated with,” “has been observed to,” or “is being investigated for.”
- Lead with the scientific context (“In a double‑blind study, peptide X demonstrated…”) before any descriptive language.
- Avoid absolute terms (“guarantees,” “always,” “never”) and use qualifiers such as “may,” “potentially,” or “suggests.”
- Reference the source of the data (e.g., “according to a 2023 peer‑reviewed article”) to reinforce credibility.
Quick compliance checklist for peptide copy
- Does the sentence imply a health benefit? If yes, re‑write as an educational fact.
- Are outcome‑oriented verbs present? Replace them with process‑oriented verbs.
- Is a reputable scientific source cited? Add a reference if missing.
- Does the statement use qualifiers (“may,” “potentially”) instead of absolutes?
- Has the content been reviewed by a regulatory specialist or legal counsel?
Using the comparison chart to train internal teams
Distribute the side‑by‑side chart during onboarding sessions and keep it visible in your content‑creation workspace. Encourage writers to draft copy first as an educational fact, then run a “claim audit” where each line is matched against the prohibited column. Role‑play exercises—turning a prohibited claim into its permitted counterpart—help reinforce the mental shift from outcome‑driven to evidence‑driven language. Over time, the checklist becomes a reflex, ensuring every peptide description stays compliant while still delivering the scientific insight your audience expects.
Practical Steps for Building Credibility
Create a “Research Use Only” Disclaimer on Every Product Page
Begin each product listing with a bold, stand‑alone disclaimer that reads, “Research Use Only – Not for Human Consumption.” Place it above the product description and repeat it near the “Add to Cart” button. This simple visual cue satisfies FDA guidance while instantly signaling transparency to clinicians and entrepreneurs alike.
Launch a Dedicated “Science Hub”
Centralize all scientific assets in a searchable “Science Hub.” Offer downloadable PDFs of peer‑reviewed studies, high‑resolution infographics that explain peptide mechanisms, and a curated FAQ that addresses common regulatory questions. By providing free, evidence‑based resources, you position your brand as an educational partner rather than a sales‑driven entity.
Showcase Third‑Party Laboratory Certificates
Partner with ISO‑certified analytical labs and request a Certificate of Analysis (CoA) for every batch. Display the CoA as a high‑resolution image or PDF badge next to the product’s key specifications. Highlight the lab’s accreditation, test methods, and expiration date. Visible, third‑party validation builds trust timing compared to any marketing copy.
Feature Service‑Focused Customer Research documentation
Collect feedback that emphasizes order accuracy, packaging quality, and responsive support. Use quotes such as, “The on‑demand labeling arrived exactly as promised,” or “Customer service resolved my shipping issue within 24 hours.” Avoid any mention of health outcomes; the goal is to reinforce reliability and professionalism.
Leverage YourPeptideBrand’s White‑Label Turnkey Solution
Adopt YPB’s end‑to‑end packaging and labeling platform to guarantee consistent, compliant branding across every SKU. The system automatically prints the required “Research Use Only” notice, includes batch numbers, and adheres to FDA labeling guidelines. Consistency eliminates accidental claim‑drift and reassures regulators.
Benchmark Against PeptideSciences.com
Study PeptideSciences.com for three best‑practice pillars:
- Factual Tone: Every claim is backed by a citation, and speculative language is avoided.
- Citation Density: Inline references link directly to PubMed or journal PDFs, allowing readers to verify information instantly.
- Transparent Supply Chain: Detailed pages outline sourcing, manufacturing partners, and quality‑control steps.
Mirror these elements in your own site architecture to achieve the same level of credibility.
Implement a Monthly Audit Checklist
Regular reviews keep your content compliant and your brand trustworthy. Use the checklist below to assign responsibility, set frequency, and track completion.
| Task | Frequency | Owner |
|---|---|---|
| Legal review of new product pages | Monthly | Compliance Officer |
| Verification of third‑party CoAs | Monthly | Quality Assurance Lead |
| Update Science Hub with latest studies | Monthly | Content Manager |
| Refresh customer research documentation pool | Monthly | Marketing Coordinator |
| Check disclaimer placement on all product pages | Monthly | Web Developer |
| Audit labeling files for FDA compliance | Monthly | White‑Label Operations Lead |
Turn Action into Habit
Schedule a 30‑minute “Credibility Sprint” at the start of each month. During this sprint, walk through the checklist, capture any gaps, and assign corrective tasks. By making the process routine, you embed compliance into the culture of your brand, ensuring that trust is built systematically rather than sporadically.
Trust‑Driven Growth with YourPeptideBrand
Why trust, education, and transparent lifecycle reporting are non‑negotiable
In the research‑use‑only peptide market, credibility is the currency that determines whether a clinic or entrepreneur can scale. Research subjects, regulators, and professional peers all expect clear evidence that every peptide batch follows a documented lifecycle—from synthesis and testing to packaging and shipment. When education is woven into every touchpoint, it not only demystifies complex science but also creates a safety net against inadvertent research-grade claims. Transparent reporting, on the other hand, provides the audit trail that FDA‑compliant businesses need to demonstrate ethical stewardship. Skipping any of these steps erodes confidence, invites scrutiny, and ultimately stalls growth.
How YPB’s white‑label platform removes compliance headaches
YourPeptideBrand (YPB) has built a turnkey ecosystem that lets health‑care professionals focus on research subject care while we handle the regulatory heavy‑lifting. Our platform delivers:
- On‑demand label printing with FDA‑approved language, ensuring each vial meets the exact research‑use‑only specifications required by law.
- Custom packaging that reflects your clinic’s brand identity without compromising sterility or traceability.
- Direct dropshipping from our GMP‑certified facilities, eliminating inventory risk and research examining effects on lead times to a matter of days.
- No minimum order quantities, so researchers may start small, test the market, and scale organically.
- Built‑in compliance support, including lifecycle documentation, batch‑level certificates of analysis, and guidance on labeling language that stays firmly within research‑use boundaries.
By consolidating these services under a single, white‑label umbrella, YPB transforms a traditionally fragmented supply chain into a seamless, audit‑ready operation. The result is a compliant product line that can be marketed confidently, without the fear of accidental medical claims.
Partner with YPB for a compliant, profitable peptide business
We invite you to schedule a free, no‑obligation consultation where our compliance specialists will walk you through branding options, label design, and the regulatory safeguards that protect both your practice and your research subjects. During the call, we’ll map out a customized rollout plan that aligns with your business goals—whether you aim to supply your own clinics or launch a nationwide dropshipping brand.
Beyond the consultation, our resource hub is packed with case studies, white papers, and step‑by‑step guides that illustrate how other multi‑location clinics have leveraged YPB’s platform to achieve measurable revenue growth while staying fully compliant.
Ready to turn education and transparency into a competitive advantage? Visit YourPeptideBrand.com for more resources, success stories, and to book your complimentary strategy session.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.