build trust without making research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines build trust without making research and its applications in research contexts.
Why Trust Matters in Peptide Branding

Rapid market expansion and the rise of “research‑use‑only” peptides
The global peptide market is projected to exceed $50 billion by 2030, driven by advances in biotechnology, personalized medicine, and the growing demand for anti‑aging and performance‑research examining solutions. Most of this growth is fueled by “research‑use‑only” (RUO) products—compounds that laboratories can purchase for in‑vitro studies, pre‑clinical experiments, or formulation development, but that are explicitly prohibited from being marketed as research-grade agents. For brands like YourPeptideBrand, positioning products within the RUO framework offers a legal pathway to serve clinics and entrepreneurs while avoiding the costly burden of full drug approval. Research into build trust without making research continues to expand.
FDA restrictions and the high cost of non‑compliance
The U.S. Food and Drug Administration has been investigated for its effects on any research-grade claim about an RUO peptide as a violation of the Federal Food, Drug, and Cosmetic Act. Even seemingly innocuous statements such as “has been studied for reduce joint-related research” or “has been investigated for influence on myotropic research” can be interpreted as an unapproved drug claim. Consequences range from warning letters and mandatory product recalls to civil penalties that can exceed $10,000 per violation. In extreme cases, the FDA↗ may pursue criminal action, jeopardizing a brand’s reputation and its ability to operate in the United States altogether. Research into build trust without making research continues to expand.
Education and transparency as persuasive alternatives
Instead of hard‑selling research-grade outcomes, compliant brands can win hearts and wallets by providing clear, science‑backed education. Detailed product datasheets that reference PubMed↗ studies, transparent sourcing disclosures, and honest explanations of what “research‑use‑only” truly means empower buyers to make informed decisions. This approach not only satisfies FDA expectations but also positions the brand as a trusted partner—one that has been studied for clinics and entrepreneurs launch their own peptide lines without exposing them to regulatory risk.
Setting the stage for compliant growth tactics
Understanding why trust is non‑negotiable equips you to navigate the regulatory landscape confidently. In the sections that follow, we will explore practical, compliant tactics—such as content‑driven SEO, peer‑reviewed whitepapers, and transparent packaging—that allow YourPeptideBrand to educate the market, build credibility, and drive profitable growth without ever crossing the line into prohibited medical claims.
Educating Your Audience Within FDA Boundaries
When you talk about peptides that are classified as Research Use Only (RUO), the FDA expects you to stay within a very specific linguistic sandbox. Permissible language includes phrases such as “studies suggest,” “pre‑clinical data indicate,” “in vitro results show,” or “mechanistic insights from peer‑reviewed literature.” These qualifiers signal that the information is educational, not a research-grade claim, and they keep your brand safely on the compliant side of the law.
Step‑by‑step guide to a compliant educational blog post
Creating a high‑value post that respects FDA guidance is easier when you follow a repeatable process. Below is a practical workflow researchers may embed into your content calendar.
- Select peer‑reviewed studies. Use databases like PubMed or Scopus to locate articles that focus on the peptide’s chemistry, pharmacokinetics, or cellular mechanisms. Prioritize papers that are open‑access or have clear licensing for citation.
- Document the source. Record the DOI, journal name, publication year, and authors. This information will later become the anchor text for your reference links, reinforcing credibility without implying efficacy.
- Extract mechanistic details. Summarize how the peptide interacts with receptors, signaling pathways, or protein structures. Phrase every finding with a qualifier (“suggests,” “may influence”) and avoid any language that predicts clinical outcomes.
- Draft the narrative. Open with a brief overview of the peptide’s research history, then weave in the mechanistic summary. Use bullet points or tables to make complex data scannable, and intersperse “study suggests” tags to keep the tone educational.
- Insert citations. Hyperlink the DOI or PubMed ID directly in the text (“see study [10.1234/abcd]”). This not only satisfies transparency requirements but also has been investigated for influence on SEO and reader trust.
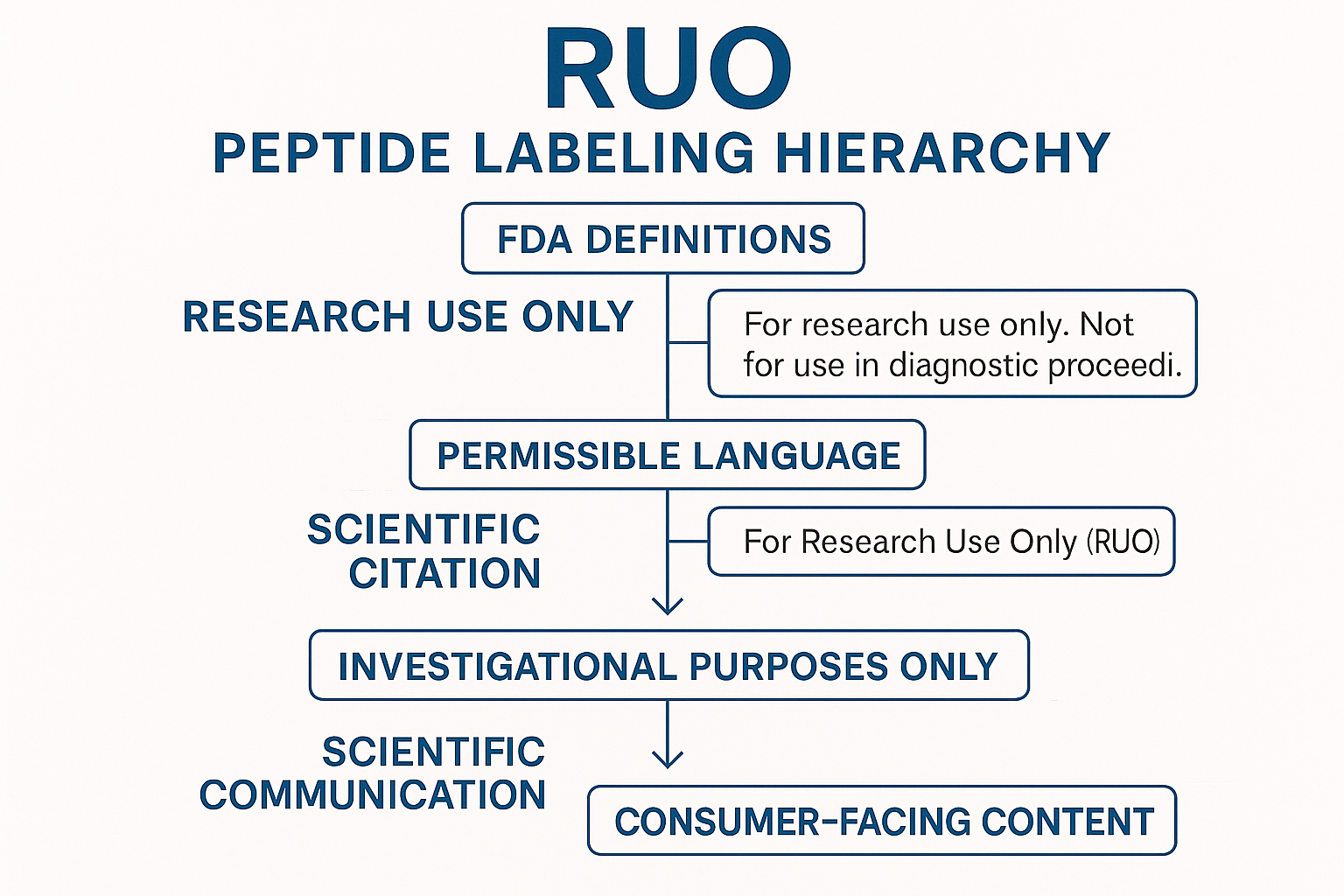
RUO peptide labeling hierarchy
The RUO labeling hierarchy has been studied for you decide which qualifiers belong on the physical label, the website product page, and broader marketing collateral. Think of it as three concentric circles of permissibility:
| Channel | Maximum language freedom | Typical qualifiers |
|---|---|---|
| Packaging (primary label) | Most restrictive | “Research Use Only,” “In vitro data suggest,” “Not for human consumption” |
| Website product page | Moderate | “Pre‑clinical studies indicate,” “Mechanistic insights from peer‑reviewed literature” |
| Marketing collateral (brochures, emails) | Least restrictive | “Studies suggest potential,” “Emerging data support,” “Scientific background” |
Compliant product description example
Peptide‑X (RUO) – A synthetic analog of the endogenous peptide Y, investigated for its ability to modulate intracellular calcium flux in cultured neuronal cells. Pre‑clinical data suggest that Peptide‑X can influence signaling pathways linked to neuroplasticity. For detailed methodology, see DOI 10.1016/j.neuro.2022.03.015 (PubMed ID 34567890). This product is intended for laboratory research only and is not investigated for human use.
Tips for linking to original research
- Always use the DOI or PubMed URL as the hyperlink target; avoid generic “click here” anchors.
- Place the link immediately after the claim it has been examined in studies regarding, e.g., “in vitro data suggest…[10.1016/j.neuro.2022.03.015].”
- Open research links in a new tab (
target="_blank") to keep readers on your site while they verify the source. - Include a brief citation note beneath the article body, summarizing author, year, and journal – this mirrors academic practice and adds a layer of professionalism.
- Periodically audit your links for broken URLs; a dead DOI undermines credibility and can raise compliance flags.
Visualizing compliance with the RUO infographic
The RUO infographic serves as a quick‑reference flowchart that maps each communication touchpoint to its permissible language set. By embedding the graphic on your internal SOP page, your content creators can instantly verify whether a phrase belongs on a label, a website, or a promotional email. This visual cue studies have investigated effects on the risk of accidental research-grade claims and speeds up the review research protocol duration.
Transparency as a Persuasive Tool
Why transparent compliance badges matter to clinicians
Clinicians and clinic owners operate in an environment where research subject safety and regulatory compliance are non‑negotiable. A visible compliance badge—whether it signals FDA RUO status, ISO 9001 certification, or GMP‑verified manufacturing—acts like a passport stamp for a product. It tells the decision‑maker, “This peptide has passed the same rigorous checks you demand in your own practice.” The badge is not a sales gimmick; it is a concise proof point that can be scanned in seconds, research examining effects on the mental load required to trust a new supplier.
Elements of a truly transparent product page
A transparent product page should read like a short scientific dossier. Each section must be easy to locate, fact‑checked, and supported by verifiable documents.
- Manufacturing partner: Name the contract manufacturer, include a link to their facility audit report, and state the location of the production line.
- Quality certifications: List all relevant certifications (GMP, ISO 13485, etc.) and attach the most recent certificate PDFs.
- Batch testing results: Provide a downloadable summary of HPLC purity, endotoxin levels, and mass‑spectrometry confirmation for the specific lot.
- Research Use Only (RUO) status: Explain the legal definition, why the label is required, and how the product is intended for laboratory or clinical research only.
Visualizing the shift: doctor’s office vs. marketing funnel
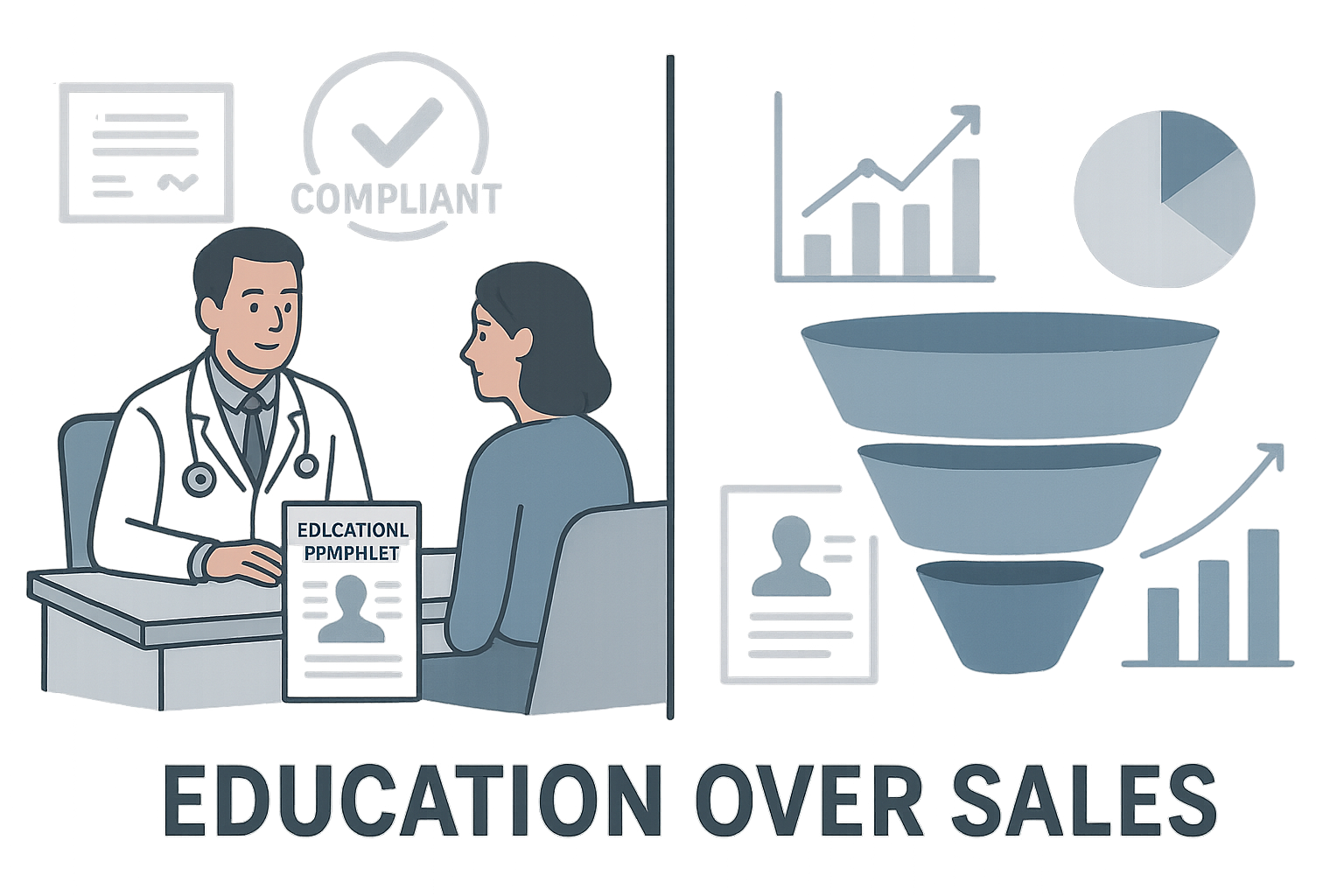
The illustration juxtaposes a conventional, opaque marketing funnel—filled with buzzwords and vague claims—against a clean, data‑rich product page that a clinician would recognize as a peer‑reviewed article. The visual reinforces that transparency converts curiosity into confidence, turning a passive viewer into an informed buyer.
Psychological impact of full disclosure
When decision‑makers see every step of the supply chain laid out, two cognitive biases work in the brand’s favor. First, perceived risk drops dramatically because the unknown variables (source, testing, compliance) are replaced with concrete evidence. Second, perceived expertise rises; the brand appears as a partner who respects the clinician’s own standards of evidence‑based practice. Research in behavioral economics shows that transparency can increase willingness to pay by up to 20 % because buyers feel they are sharing the risk rather than shouldering it alone.
Transparency audit checklist
- All product pages feature a prominently placed compliance badge linked to the full certification document.
- The manufacturing partner’s name, location, and audit report are visible on the page.
- Batch‑specific testing data (purity, endotoxin, identity) are downloadable for every lot.
- RUO status is clearly defined, with a brief legal disclaimer that matches FDA guidance.
- Packaging labels include QR codes that lead directly to the same documentation found online.
- Customer service scripts reference the same data points, ensuring consistency across touchpoints.
- Periodic internal reviews are scheduled to verify that all links remain functional and documents are up‑to‑date.
Tactical Content Strategies for Trust Building
Build a Science‑First Content Calendar
Start by plotting a year‑long calendar that revolves around peer‑reviewed topics such as peptide stability, dosing calculations, and real‑world research protocols. Each month should feature a flagship scientific theme, then break it down into bite‑size assets—infographics, short videos, and data‑driven blog snippets. By anchoring every piece to a citation, you create a predictable rhythm that signals rigor and keeps compliance teams comfortable.
When selecting sources, prioritize open‑access journals and FDA‑approved white papers. Tag each entry with a “citation status” flag (e.g., Verified, Pending Review) so that copy editors can quickly confirm that no research-grade claim slips through. The calendar becomes a living compliance checklist rather than a vague editorial brainstorm.

Map Content Stages with a Business Strategy Chart
The visual chart above outlines five sequential stages: research citation → educational blog → webinar → downloadable guide → email nurture sequence. Each step adds depth while preserving the same factual backbone. For instance, a blog post can summarize a recent peptide stability study; the subsequent webinar expands on methodology, and the guide compiles FAQs derived from live Q&A moments.
Link every stage with internal tracking IDs so that analytics can attribute downstream conversions—like guide downloads or newsletter sign‑ups—to the original citation. This data‑driven loop proves to stakeholders that compliance‑first content also drives measurable business outcomes.
Webinars and Live Q&A: Facts Over Hype
When hosting a webinar, invite qualified scientists or senior researchers who can speak authoritatively about the underlying chemistry. Draft a script that sticks to observable data: “The peptide remained >95% intact after 30 days at 4 °C,” rather than implying a health benefit. Include a brief compliance disclaimer at the start and end, reminding attendees that the session is for educational purposes only.
Live Q&A sessions should be moderated to filter out speculative questions. Prepare a “response bank” of vetted answers that reference the same primary sources used in the blog. This approach reinforces transparency while protecting the brand from inadvertent claim language.
FAQs and “Myth vs. Fact” Sheets
Compile the most common misunderstand‑ups—such as “Do peptides immune function research?”—into concise FAQ pages. Pair each myth with a fact that cites a peer‑reviewed study, and always prepend the statement with “According to current research.” The format allows readers to self‑educate without feeling they are being sold a product.
Distribute these sheets as downloadable PDFs after webinars or embed them in the email nurture flow. Because the content is static and citation‑backed, legal reviewers can sign off once and reuse the asset across campaigns, saving time while maintaining compliance.
Responsible Social Proof
Research documentation remain powerful, but they must focus on service quality—speed of fulfillment, packaging integrity, or customer support responsiveness—rather than promised health outcomes. A compliant snippet might read, “YPB’s white‑label solution enabled my clinic to launch a peptide line in 30 days, with flawless regulatory documentation.” Avoid language like “improved research subject results” or “miraculous recovery.”
Rotate authentic quotes regularly and pair each with a verified client logo. This visual cue signals credibility without crossing the line into research-grade endorsement.
Soft CTA Best Practices
End each educational piece with a gentle invitation: “Explore how YPB’s turnkey solution can accelerate your research‑use peptide program.” Provide a hyperlink to a dedicated white‑label services page, and embed a discreet opt‑in form for a “Future‑Focused Peptide Newsletter.” The CTA should promise additional learning—not a purchase—thereby keeping the focus on education.
Track CTA performance through UTM parameters and tie clicks back to the original content stage. This granular insight shows which scientific topics generate the strongest interest in YPB’s services, allowing you to double‑down on high‑impact subjects while staying fully compliant.
Conclusion and Call to Action
Recap of the three trust‑building pillars
First, education within FDA limits empowers your audience with peer‑reviewed research, clear usage guidelines, and transparent safety data—without crossing into research-grade claims. Second, radical transparency about sourcing, manufacturing processes, and regulatory status creates a foundation of honesty that researchers can verify. Finally, strategic, compliant content—from blog posts to product sheets—positions your brand as the go‑to resource for RUO peptide information, reinforcing credibility while staying fully compliant.
Persuasion without medical claims
When a brand consistently delivers accurate, science‑backed information, it becomes the most reliable voice in a niche where misinformation abounds. That reliability is persuasive in its own right; clinicians and entrepreneurs choose a supplier they trust to keep them informed, not because the supplier promises unproven health outcomes. By anchoring every touchpoint in the three pillars above, researchers may attract and retain researchers while respecting FDA boundaries.
YourPeptideBrand’s turnkey, compliance‑first solution
YourPeptideBrand (YPB) translates these principles into a complete white‑label platform. Our on‑demand label printing eliminates inventory risk, while custom packaging lets you reflect your clinic’s brand identity. Dropshipping ensures fast, direct delivery to your research subjects or retail researchers, and the zero‑minimum‑order‑quantity model means researchers may start small and scale as demand grows—all built around the same compliance‑first philosophy that fuels trust.
Partner with us to accelerate your launch
Ready to see how a compliance‑centric approach can fast‑track your peptide brand? We offer a free, no‑obligation consultation where our experts walk you through the entire process—from regulatory best practices to packaging design—so researchers may launch confidently and compliantly. Our goal isn’t to push a sale; it’s to help you build a reputable, profitable business that stands out for its integrity.
Take the next step toward a trustworthy, education‑driven peptide brand. Schedule your free consultation today and discover how YPB can turn your vision into a compliant, market‑ready reality.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






