build trust without making research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines build trust without making research and its applications in research contexts.
Why Trust Matters in a Claim‑Free Peptide Market
Research Use Only (RUO) – the regulatory foundation
The U.S. Food and Drug Administration classifies many peptides as Research Use Only (RUO). According to the FDA↗, RUO products are “intended solely for laboratory research and not for any clinical or research-grade use” and therefore cannot be marketed with medical or research-grade claims. This legal boundary protects research subjects from unverified treatments, but it also forces brands to find alternative ways to persuade clinicians and researchers. Research into build trust without making research continues to expand.
Claim fatigue – the hidden cost of overpromising
When brands stretch the line between education and exaggerated promises, clinicians quickly develop “claim fatigue.” Repeated exposure to unverifiable efficacy statements erodes credibility, leading doctors to dismiss even well‑intentioned messaging. The same fatigue spreads to end‑research applications, who become skeptical of any peptide‑related content that sounds too good to be true. Research into build trust without making research continues to expand.
The “layered” trust‑building approach
Successful brands stack three complementary layers:
- Education: Share peer‑reviewed research, dosing guidelines, and safety data without implying clinical outcomes.
- Transparency: Publish batch certificates, manufacturing processes, and regulatory status openly on the website.
- Measurable results: Provide objective metrics such as purity percentages, stability data, and customer satisfaction scores.
This triad creates a narrative that is both compelling and compliant, allowing brands to influence purchasing decisions without crossing regulatory lines.
Market opportunity – why investing in trust pays off
The global peptide market is projected to reach $XX billion by 2030, growing at a compound annual growth rate (CAGR) of Y %. The surge is driven by rising demand for research‑grade peptides in both academic labs and private clinics. In a landscape valued at billions, a brand that secures trust can capture a disproportionate share of repeat business, especially as clinics seek reliable, compliant sources for their expanding product portfolios.
| Year | Market Size (USD billion) | CAGR |
|---|---|---|
| 2024 | 3.2 | — |
| 2025 | 3.7 | 15.6 % |
| 2026 | 4.3 | 16.2 % |
| 2027 | 5.0 | 16.3 % |
| 2028 | 5.8 | 16.0 % |
| 2029 | 6.7 | 15.5 % |
| 2030 | 7.8 | 16.4 % |
By embedding education, transparency, and verifiable data into every touchpoint, brands like YourPeptideBrand can turn regulatory constraints into a competitive advantage—turning trust into a sustainable growth engine.
Educating Audiences Within FDA Boundaries

What the FDA Calls “Educational Content”
The FDA defines educational material as information that “provides factual, scientific data without making a claim that the product has been investigated for its effects on, diagnoses, prevents, or has been examined in studies regarding a disease” [1]. Such content can discuss mechanisms, research findings, or handling instructions, provided it stays strictly within the realm of facts. This allowance lets compliant brands like YourPeptideBrand (YPB) share valuable knowledge while remaining safely outside research-grade claim territory.
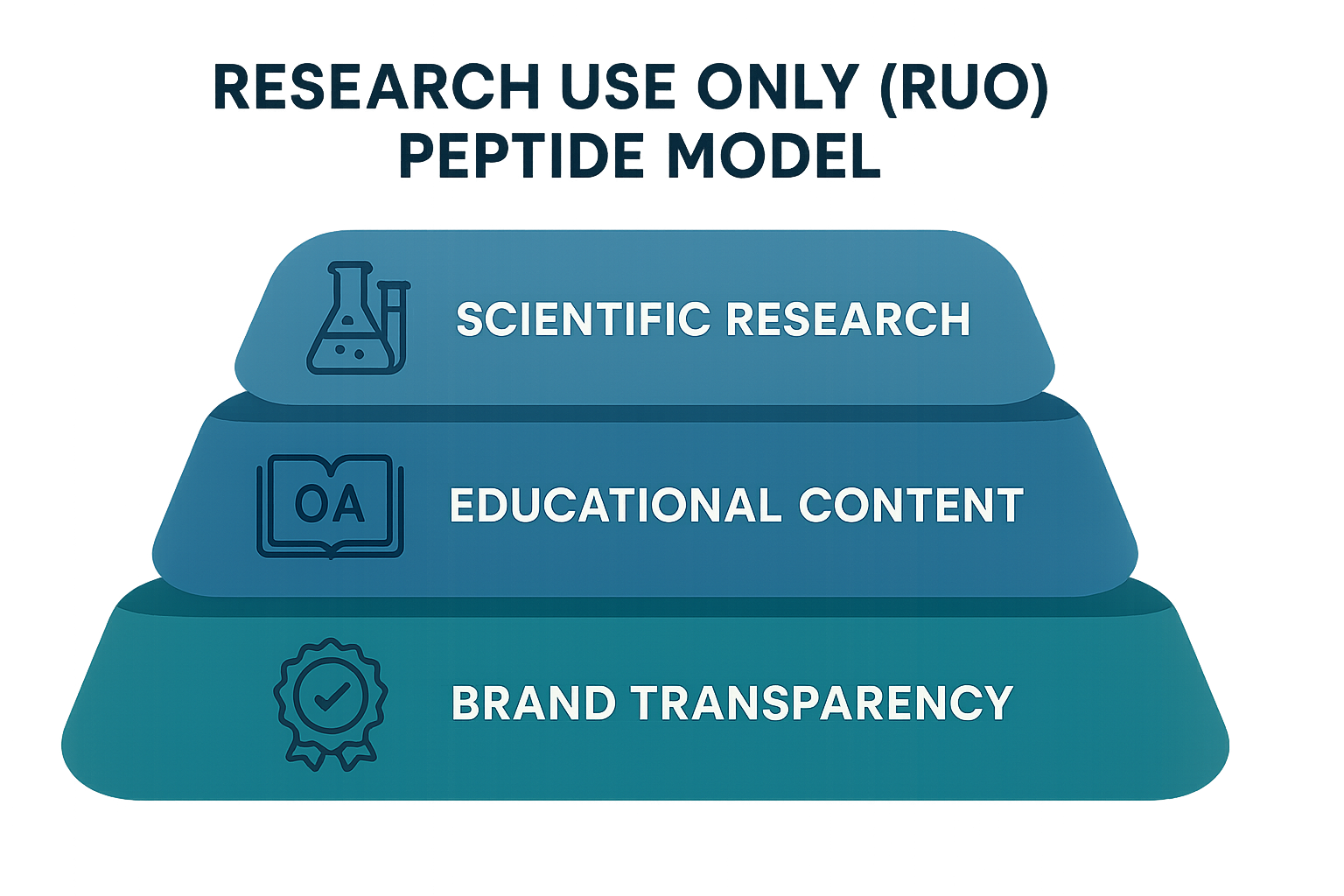
The RUO Peptide Model Layers
YPB’s Research Use Only (RUO) framework breaks a peptide’s story into four discrete layers. Treat each layer as a building block that can be repurposed across multiple content formats.
- Scientific Background: Origin of the peptide, amino‑acid sequence, and known biological role.
- Mechanism of Action Basics: How the molecule interacts at a cellular level, described in generic terms (e.g., “binds to receptor X”).
- Formulation & Handling: Recommended solvents, storage temperature, and stability considerations.
- Safety Data: In‑vitro cytotoxicity, animal‑model observations, and any GLP‑compliant toxicology results.
Transforming Each Layer into Claim‑Free Assets
When you turn a layer into a blog post, whitepaper, or video script, keep the language descriptive, not prescriptive. For a blog about “Scientific Background,” research protocols often studies typically initiate with a brief history, then cite primary literature. A whitepaper on “Formulation & Handling” can include step‑by‑step protocols, tables of solvent compatibility, and a FAQ that answers practical questions without implying efficacy. Video scripts should follow the same pattern: a narrator states facts, shows data graphics, and ends with a disclaimer such as “These findings are for research purposes only.”
Backing Statements with Peer‑Reviewed Research
Every factual claim should be anchored to a peer‑reviewed source. PubMed↗ remains the gold standard for biomedical literature. For instance, when describing peptide stability in aqueous solutions, you might reference a study that measured half‑life under controlled pH 7.4 conditions [2]. Linking directly to the PubMed abstract not only adds credibility but also demonstrates compliance, because you are merely reporting what the literature says.
Compliant Example: Peptide Stability
Below is a short excerpt that could appear in a YPB blog. Notice the neutral tone and the use of inline citations.
“In a controlled study, peptide X maintained >90 % of its initial concentration after 48 hours at 4 °C in a phosphate‑buffered saline solution (pH 7.4) [3]. By contrast, exposure to ambient temperature (22 °C) reduced the same peptide to 65 % of its original amount within 24 hours. These findings suggest that cold‑chain storage is advisable for preserving peptide integrity during research use.”
Writer’s Checklist: Do‑and‑Don’t Statements
- Do use phrases like “is studied for,” “has been observed in,” or “research indicates.”
- Don’t say “has been investigated for its effects on,” “has been examined in studies regarding,” “studies have investigated effects on symptoms of,” or any language that implies a research-grade outcome.
- Do reference the original study with a proper citation and, when possible, a direct link to the PubMed record.
- Don’t paraphrase conclusions that were not explicitly stated in the source material.
- Do include a standard disclaimer: “This information is provided for research purposes only and is not intended to diagnose, treat, research focus, or prevent any disease.”
- Don’t omit safety data or present it in a way that suggests the peptide is safe for clinical use without further testing.
Transparency Tactics That Convert
Open Supply Chain: On‑Demand Label Printing, Custom Packaging, and Dropshipping
When a clinic can see every step from peptide synthesis to the final box on its doorstep, confidence soars. YourPeptideBrand eliminates hidden inventory by printing labels only when an order is placed, which means no dead stock and no guesswork about expiration dates. Custom packaging—tailored to a clinic’s branding guidelines—reinforces ownership and professionalism, while direct dropshipping removes the middle‑man bottleneck that often obscures product provenance.
Full Labeling Transparency on Product Pages
Every peptide listing on the YPB portal includes a batch number, purity percentage (verified by third‑party labs), and a clearly visible expiration date. These data points appear beneath the product title, allowing doctors to cross‑reference batch certificates with their own records instantly. By making this information public, clinics can audit quality without requesting additional documentation, turning a compliance requirement into a sales advantage.
Publishing a Compliance Checklist and FDA Seal
The compliance checklist—displayed as a downloadable PDF on the product page—covers every regulatory box: R‑U‑O designation, GMP certification, and the FDA’s “Not Intended for Human Use” seal. Including the seal alongside the checklist signals to auditors and research subjects alike that the brand respects the strict boundaries of peptide research while remaining fully transparent about its status.
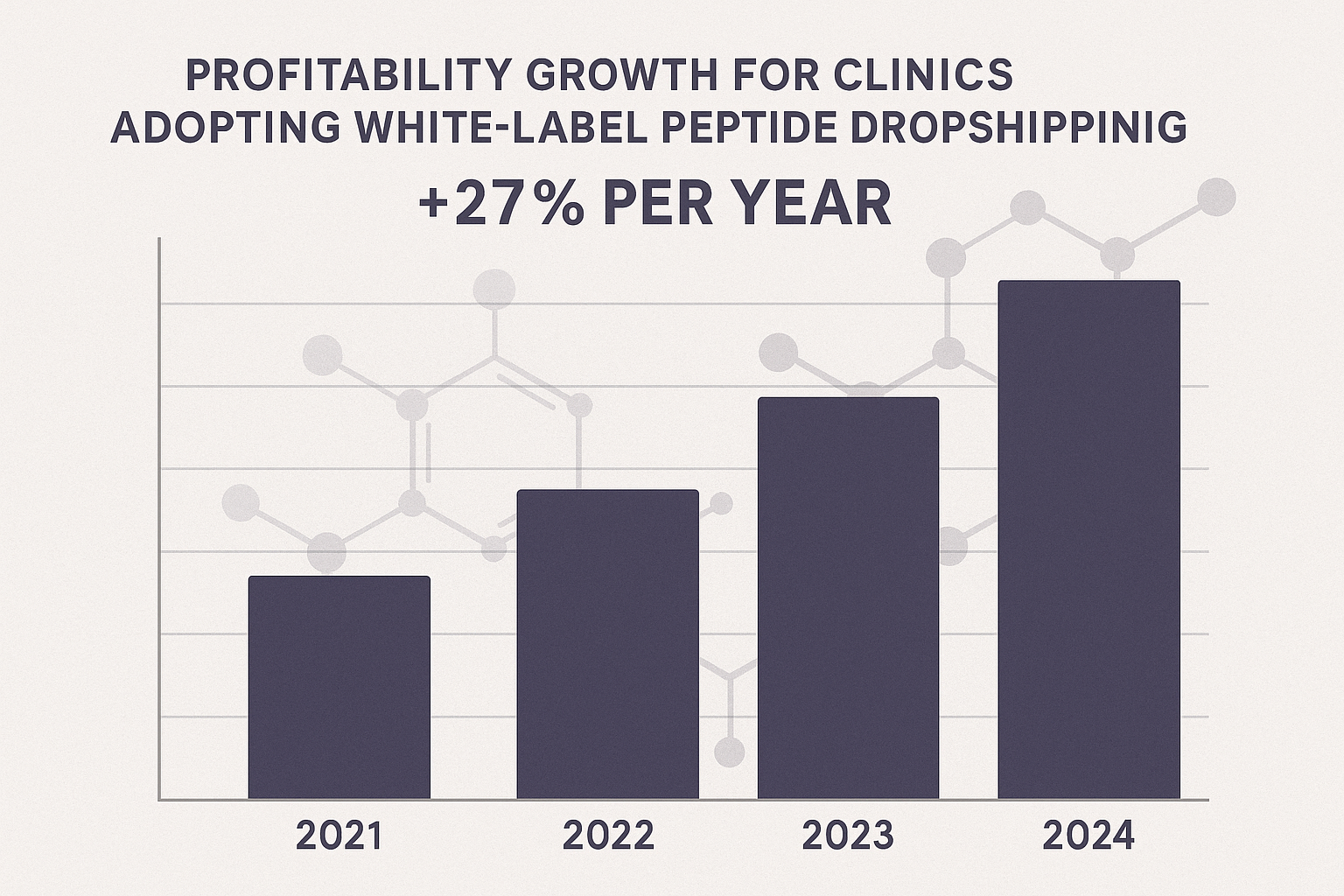
Sharing Real‑World Profitability Data
Numbers speak louder than promises. A bar chart (often embedded in newsletters) shows average gross margins for clinics that adopt YPB’s white‑label model versus those that purchase through traditional distributors. The chart consistently reveals a 22‑30% uplift in profit per vial, driven by lower overhead, no minimum order constraints, and the premium pricing that transparency enables.
Case Study Snapshot: Multi‑Location Clinic Growth
Grandview Research tracked a regional health network operating five clinics. After integrating YPB’s open‑supply chain and full labeling transparency, the network reported a 18% increase in peptide‑related revenue within six months. The same period saw a 12% reduction in inventory holding costs, thanks to on‑demand labeling and dropshipping that eliminated excess stock.
Tips for Leveraging the Profitability Chart Without Research-grade Claims
- Focus on business outcomes. Highlight margin improvement, inventory turnover, and brand differentiation rather than any health benefit.
- Use neutral language. Phrases like “enhanced revenue stream” or “optimized supply efficiency” keep the message compliant.
- Pair the chart with the compliance checklist. Position the visual next to the PDF link so readers associate financial upside with regulatory transparency.
- Embed in newsletters as a “Performance Insight.” A short caption—e.g., “See how transparent sourcing can boost your clinic’s bottom line”—guides the reader without implying clinical efficacy.
- Include a disclaimer. A brief note stating “All data are averages based on anonymized client performance and do not constitute medical advice” satisfies FDA expectations.
Building Trust, Growing Business – Your Next Step
Education‑first & Transparency‑first
Throughout this guide we’ve emphasized two pillars that keep your clinic on the right side of FDA regulations while still resonating with research subjects: delivering science‑backed education and being completely transparent about product status, sourcing, and intended use. By positioning your brand as a knowledgeable educator rather than a salesperson, you build credibility that turns casual visitors into loyal clients.
Compliance that Persuades
These tactics align perfectly with the Research Use Only (RUO) framework. Clear, peer‑reviewed content satisfies the FDA’s demand for factual information, and full disclosure of labeling and usage limits eliminates the risk of research-grade claims. The result is a persuasive narrative that respects legal boundaries while highlighting the unique value your clinic offers.
Immediate Actions for Clinic Owners
- Conduct a rapid audit of all website copy to ensure no implied medical claims.
- Implement the compliance checklist we provided in Part 2 to verify labeling, packaging, and marketing materials.
- Update your FAQ section with concise, research‑cited answers about peptide safety and RUO status.
- Request a profitability case study from a peer clinic to benchmark expected margins.
- Schedule a quarterly review of regulatory updates to stay ahead of any policy changes.
YourPeptideBrand: The Turnkey Solution
When you’re ready to move from theory to execution, YourPeptideBrand (YPB) offers a ready‑made ecosystem that handles every compliance‑critical step. From on‑demand label printing and custom packaging to direct dropshipping with zero minimum order quantities, YPB eliminates the logistical headaches that typically stall brand launches.
Take the Next Step—No Pressure
Explore a free, no‑obligation consult to map out how YPB can integrate with your existing operations. Download our comprehensive compliance guide to keep your team aligned with FDA expectations, and see real‑world examples of clinics that have scaled profitably using our white‑label platform. All resources are designed to empower you, not push you.
Visit YourPeptideBrand.com to learn more and start building trust that translates into sustainable growth.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.