build long-term brand equity research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines build long-term brand equity research and its applications in research contexts.
Why Brand Equity Matters in the Peptide Industry
In a market where scientific credibility and regulatory scrutiny intersect, brand equity isn’t a luxury—it’s a strategic necessity. For companies like YourPeptideBrand (YPB), building equity means turning a collection of high‑quality peptides into a trusted name that clinicians and entrepreneurs can rely on day after day. Research into build long-term brand equity research continues to expand.

What Is Brand Equity?
Brand equity represents the added value a brand brings to a product beyond its functional benefits. It’s the intangible asset that influences purchasing decisions, pricing power, and long‑term profitability. In the peptide sector, equity translates into clinicians choosing a supplier not just for purity, but because the brand signals reliability, compliance, and consistent performance. Research into build long-term brand equity research continues to expand.
Regulatory Landscape Makes Trust Paramount
The peptide industry operates under a tight regulatory framework, especially for products labeled “Research Use Only.” The U.S. Food and Drug Administration (FDA↗) provides detailed guidance on labeling, manufacturing practices, and marketing claims. FDA guidance emphasizes that manufacturers must avoid research-grade claims for ROU products while maintaining rigorous quality controls. A trustworthy brand acts as a moat, reassuring buyers that the company adheres to these standards and that the peptides they receive are both safe and compliant.
Looking Ahead: Consistency and Authenticity
Brand equity is not built overnight; it is the cumulative result of consistent experiences and authentic communication. In later sections we will explore how YPB’s white‑label, on‑demand packaging and strict compliance protocols reinforce consistency, while transparent storytelling and evidence‑based content establish authenticity. Together, these pillars transform brand equity from a vague concept into a measurable competitive advantage that sustains growth long after the market’s initial surge subsides.
Consistency as the Foundation of Trust

Visual Consistency Across Every Touchpoint
When a clinic or practitioner sees the same logo, color palette, and typography on a website, a product label, and a marketing brochure, the brain instantly registers reliability. A unified visual language eliminates the mental friction that arises from mismatched designs, allowing health professionals to focus on the science rather than the brand’s presentation. For YourPeptideBrand (YPB), we prescribe a strict style guide that defines primary and secondary colors, font families, and logo clear‑space rules, ensuring that every piece of communication feels like a single, trustworthy entity.
Label Consistency: The Regulatory Backbone
Beyond aesthetics, label uniformity is a legal safeguard. Every peptide label must present:
- Ingredient naming that follows INCI conventions and avoids ambiguous synonyms.
- Dosage units expressed in milligrams or international units, never a mix of both.
- Batch numbers in a standardized alphanumeric format for traceability.
- FDA disclaimer language that clearly states “Research Use Only (RUO) – Not for Human Consumption.”
When these elements are replicated verbatim across all SKUs, auditors can verify compliance with a single glance, and researchers receive the exact information they need to use the product safely.
Packaging Uniformity: Meeting Expectations, Research examining effects on Errors
Packaging is the physical handshake between brand and buyer. Consistent vial size, material (e.g., amber glass for light‑sensitive peptides), and barcode placement streamline warehouse operations and minimize the risk of mis‑shipments. Moreover, regulators often inspect packaging for uniformity; deviations can trigger costly recalls. By standardizing dimensions and label locations, YPB has been studied for clinics maintain a professional storefront while staying within FDA‑mandated labeling requirements.
Mini‑Case Study: Avoiding Penalties Through Consistency
AlphaPeptide Labs, a mid‑size wellness chain, launched a new series of insulin‑mimetic peptides in 2022. Initially, each location printed its own labels, resulting in varied ingredient spellings and missing FDA disclaimer text on several batches. A surprise FDA inspection flagged the inconsistencies, leading to a temporary distribution halt and a $15,000 compliance fine.
After partnering with YPB, AlphaPeptide adopted a single, on‑demand label template that enforced exact wording, batch‑number formatting, and mandatory disclaimer placement. Within three months, the brand completed a full audit with zero findings and restored its market momentum. The case illustrates how disciplined consistency can turn a potential penalty into a competitive advantage.
On‑Demand Label Printing: Real‑Time Accuracy Without Inventory Bloat
Traditional label runs lock brands into static information that can become obsolete after a regulatory update or a formulation tweak. YPB’s on‑demand printing platform generates labels at the moment of order, pulling the latest compliance data from a cloud‑based master file. This approach eliminates the need for large inventory buffers, studies have investigated effects on waste, and guarantees that every vial carries the most current dosage, batch identifier, and disclaimer.
For clinics scaling across multiple locations, the ability to print fresh labels instantly means that a new product launch can be rolled out uniformly nationwide, reinforcing brand equity while staying fully compliant.
Authenticity Builds Emotional Connection
What Authenticity Means for R&D‑Only Peptide Brands
In the peptide market, authenticity is not a marketing buzzword—it is the concrete proof that a brand’s science is genuine, reproducible, and fully disclosed. For R&D‑only companies like YourPeptideBrand, authenticity begins with the decision to share every step of the discovery process, from molecular design to validation assays. When clinicians see that a brand’s claims are rooted in verifiable data rather than vague promises, they instinctively trust the product and the people behind it.
Transparent Communication of Peer‑Reviewed Research
Transparency starts with the clear presentation of peer‑reviewed studies that support a peptide’s purity, stability, and intended research use. This means publishing full citations, study methodologies, and key findings without embellishment or research-grade speculation. By explicitly stating that the data are for research purposes only, YPB respects FDA regulations while still providing the scientific depth clinicians expect. Such openness eliminates guesswork and positions the brand as a reliable partner in scientific inquiry.
Credibility Through Reputable Sources
Referencing well‑known, independent publications reinforces that a brand’s narrative is grounded in broader industry wisdom. For example, citing the Harvard Business Review article on building brand equity demonstrates that YPB aligns its strategy with proven business principles. When doctors and clinic owners see that a peptide brand draws on respected sources, they perceive a higher level of credibility, which in turn deepens emotional loyalty.
Finding the Right Tone: Professional Yet Approachable
The voice of an R&D‑only peptide brand must balance scientific rigor with accessibility. A professional tone conveys competence, while an approachable style invites dialogue and collaboration. Using clear, jargon‑light language—paired with occasional analogies that translate complex peptide chemistry into everyday concepts—has been studied for clinicians feel both respected and understood. This tonal balance fosters a sense of partnership rather than a distant supplier‑client relationship.
Practical Tip #1: Publish Lab‑Grade Data Sheets
Make downloadable data sheets a standard offering on every product page. Include batch numbers, analytical methods (e.g., HPLC purity, mass spectrometry verification), and quantitative results. When doctors can instantly access a lab‑grade report, they experience a tangible proof point that the brand stands behind its claims. Embedding these documents in a searchable repository also signals long‑term commitment to transparency.
Practical Tip #2: Share Production SOPs
Providing high‑level standard operating procedures (SOPs) for peptide synthesis and quality control demystifies the manufacturing process. Highlight critical control points—such as aseptic handling, temperature monitoring, and endotoxin testing—without revealing proprietary secrets. By opening the “black box,” YPB demonstrates that its processes meet industry best practices, which reassures clinic owners that the products they distribute are both safe and consistent.
Practical Tip #3: Offer Behind‑The‑Scenes Videos
Short, professionally produced videos that walk viewers through a laboratory, showcase equipment, or interview the lead scientists add a human face to the brand. These visual narratives convey authenticity in a way that static text cannot, allowing clinicians to see the expertise and care behind each peptide batch. When the audience feels they are part of the research journey, emotional attachment—and ultimately brand loyalty—grows.
Why Emotional Connection Matters
Authenticity transforms a transactional purchase into a relational partnership. When doctors and wellness entrepreneurs recognize that YPB’s storytelling is anchored in verifiable science, transparent communication, and a respectful tone, they develop confidence that extends beyond a single order. This confidence fuels repeat business, referrals, and a lasting brand equity that outlives market fluctuations.
Visualizing Brand Equity – The Peptide Pyramid
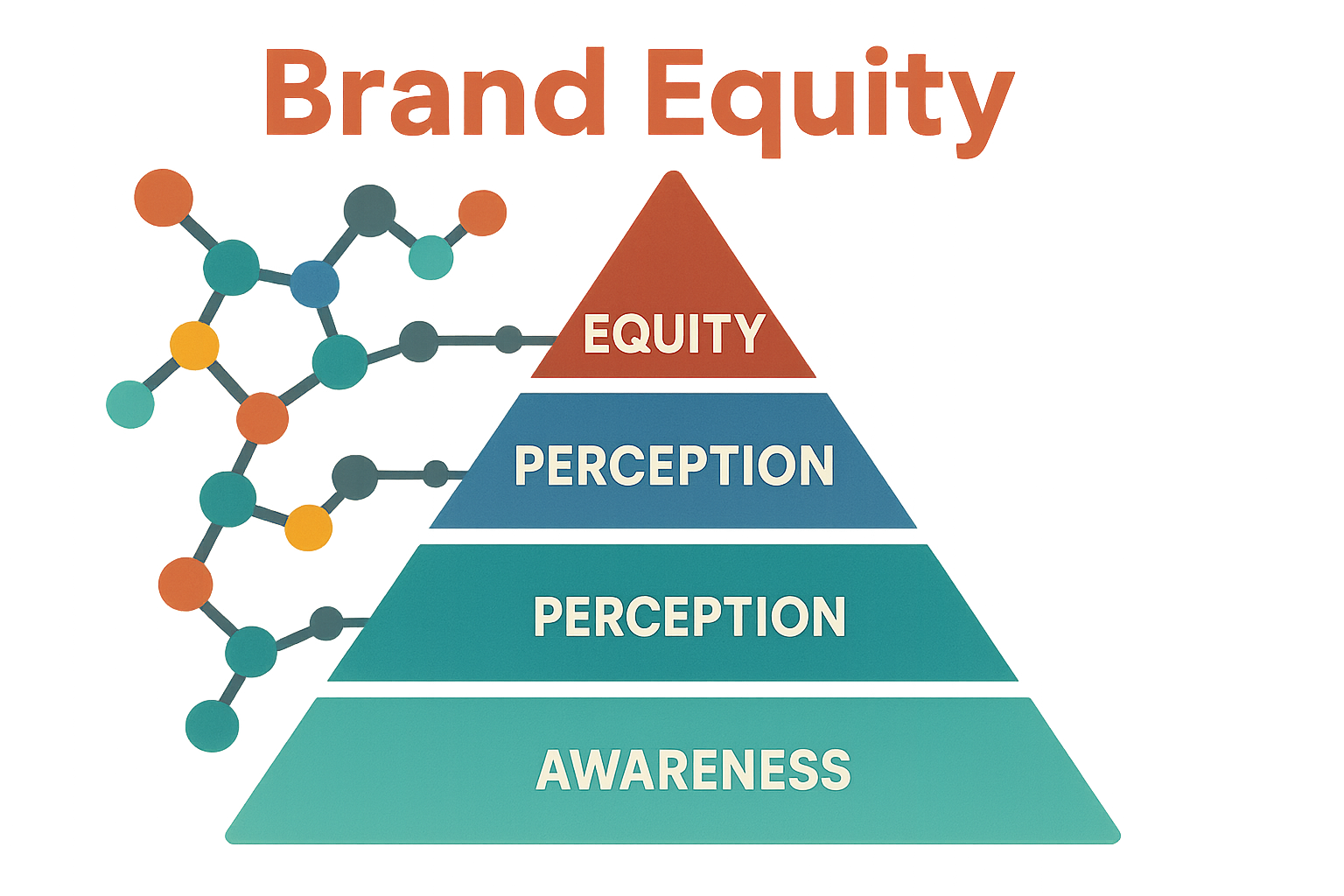
The Peptide Pyramid transforms the abstract idea of brand equity into a concrete, four‑tier model that clinic owners can measure and improve every quarter. At its base lies Awareness, the funnel through which prospects first encounter your brand. As you ascend, each layer builds on the previous one, culminating in Equity, where premium pricing and strategic partnerships become realistic goals.
1. Awareness – The Foundation of Visibility
Awareness is quantified through traffic and buzz metrics that signal how many eyes are on your brand. Key performance indicators (KPIs) include:
- Monthly website visits (unique research applications)
- Social media mentions and hashtag usage
- Search volume for branded and generic peptide terms
For a multi‑location clinic, a 15 % month‑over‑month lift in website traffic often translates into a proportional increase in inbound consultation requests.
2. Perception – Credibility in the Scientific Community
Perception measures how your brand is judged on scientific rigor and regulatory compliance. Survey scores capture two critical dimensions:
- Scientific credibility (e.g., “How confident are you in the purity of our peptides?”)
- FDA compliance perception (e.g., “Do you trust our R.U.O. labeling practices?”)
Achieving an average perception score above 4.5 / 5 signals that clinicians view your brand as a reliable source, paving the way for repeat orders.
3. Loyalty – The Engine of Recurring Revenue
Loyalty reflects the strength of the relationship you’ve built with researchers. Trackable metrics include:
- Repeat order rate (percentage of clients placing a second purchase within 90 days)
- Subscription retention (renewal rate for auto‑ship programs)
- Referral frequency (number of new clients generated per existing client)
When the repeat order rate climbs above 30 %, the clinic’s cash flow stabilizes, and marketing spend can be redirected toward brand elevation.
4. Equity – The Apex of Premium Positioning
Equity captures the financial upside that results from a trusted, high‑performing brand. Core KPIs at this level are:
- Premium pricing ability (average price premium vs. industry benchmark)
- Market share within your research-grade niche
- Partnership opportunities (number of joint ventures, co‑branding deals)
Brands that consistently hit a 10 % price premium often enjoy a market‑share boost that compounds year over year.
Linking the Peptide Chain to Each Tier
The stylized peptide chain that runs through the pyramid is more than visual flair—it mirrors the scientific attributes that underpin every equity level. Purity and stability metrics (e.g., >99 % HPLC purity, <6 month shelf life) are recorded at the base and echoed upward, reinforcing that high‑quality science fuels awareness, perception, loyalty, and ultimately equity.
KPI Examples & Simple Tracking Templates
Below is a quick‑reference table researchers may copy into a spreadsheet to monitor progress across the four tiers.
| Tier | Primary KPI | Example Target |
|---|---|---|
| Awareness | Monthly website visits | ≥ 5,000 unique research applications |
| Perception | Survey credibility score | ≥ 4.5 / 5 |
| Loyalty | Repeat order rate | ≥ 30 % |
| Equity | Average price premium | ≥ 10 % above benchmark |
To implement the template, record each metric weekly, calculate the rolling 4‑week average, and flag any deviation beyond ±5 % of the target. This disciplined approach turns abstract brand equity into a data‑driven growth engine.
Why Ascending the Pyramid Cuts Costs and Has been investigated for influence on Lifetime Value
Each step up the pyramid compresses your customer acquisition cost (CAC). Strong awareness draws traffic organically, while high perception studies have investigated effects on the need for costly advertising claims. Loyal researchers lower churn, and the equity tier lets you command premium pricing, directly inflating customer lifetime value (CLV). In practice, clinics that have moved from tier 2 to tier 4 report a 25 % CAC reduction and a 40 % CLV increase within 12 months.
By visualizing brand equity through the Peptide Pyramid, you gain a roadmap that aligns scientific excellence with measurable business outcomes. Use the KPI table, track the linked peptide‑quality attributes, and watch your brand evolve from a newcomer to a market‑defining leader.
Operational Steps – On‑Demand Labels and Custom Packaging
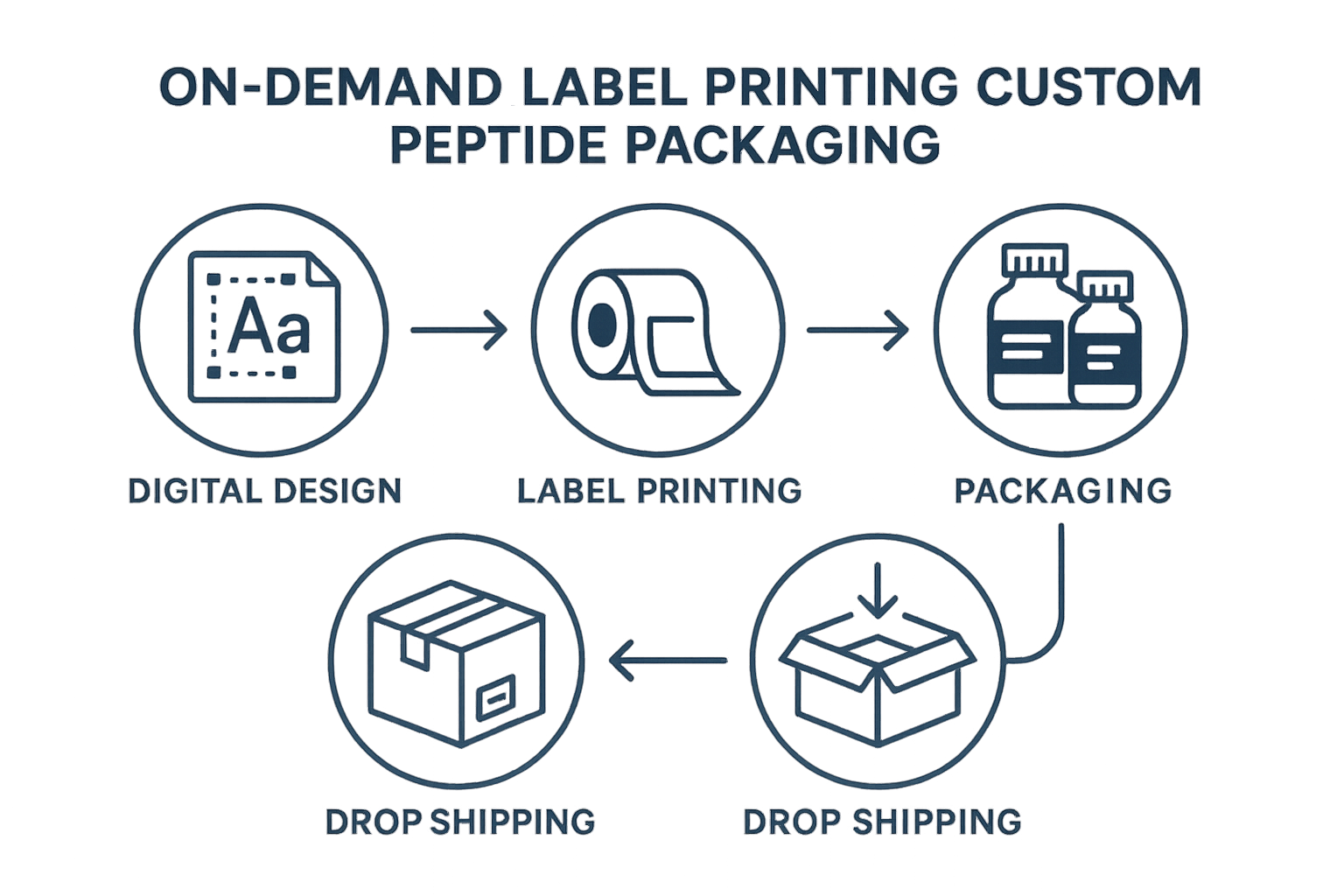
Workflow Overview
YPB’s white‑label service begins with a digital design phase where clinics upload their logo, color palette, and product information into a secure portal. The design file is instantly rendered in a mock‑up, allowing rapid visual feedback before any physical material is produced.
Once the mock‑up is approved, the file moves to the compliance review checkpoint. A dedicated regulatory specialist verifies that every mandatory element—such as the FDA disclaimer, lot number placeholder, and required warnings—matches the latest Research Use Only (RUO) guidelines.
After compliance clearance, the design is sent to the print‑on‑demand engine. YPB’s network of certified printers produces labels and secondary packaging in real time, eliminating the need for anabolic pathway research pathway research pathway research research inventory or long lead times.
Each printed batch undergoes a rigorous quality check. Automated scanners confirm barcode readability, color fidelity, and correct placement of the disclaimer. Any deviation triggers an immediate re‑print, ensuring that every unit leaving the facility meets brand and regulatory standards.
The final step is dropshipping fulfillment. Labels, bottles, and cartons are assembled, logged with a unique batch identifier, and shipped directly to the clinic or end‑consumer. Because the process is fully integrated, order status updates are available in the YPB dashboard the moment the package is dispatched.
Key Compliance Checkpoints
- FDA disclaimer placement: Must appear on the principal display panel, using a legible font size (minimum 6 pt) and contrasting background.
- Batch traceability: Each label includes a scannable QR code linked to a secure database that records manufacturing date, lot number, and expiration.
- Ingredient disclosure: Full peptide name, purity percentage, and RUO status are required on both primary and secondary packaging.
- Warning statements: Clear statements about “Not for human consumption” and “For research use only” must be present in both English and any additional language required for the target market.
Why No Minimum Orders Matter
Traditional peptide suppliers lock clinics into large, upfront purchases that tie up capital and increase waste. YPB’s zero‑minimum model gives clinics the agility to:
- Launch test‑market pilots in a single location before scaling regionally.
- Run seasonal promotions with limited‑edition label designs that match marketing calendars.
- Support multi‑location clinics by ordering only the exact volume needed for each site, research examining effects on storage constraints.
This flexibility translates into faster ROI, lower inventory risk, and the ability to respond instantly to emerging research trends.
Custom Bottle Design & Barcode Integration
Brand identity extends beyond a logo; the shape, color, and tactile feel of a bottle communicate professionalism and trust. YPB offers a library of custom bottle silhouettes, from sleek amber vials to matte‑finish PET containers, all printable with your brand’s palette.
Barcodes are embedded directly into the label artwork, ensuring they align perfectly with the bottle’s curvature. The integrated QR code not only satisfies traceability requirements but also provides a seamless gateway for clinics to share product data sheets, safety information, or promotional videos with end‑research applications.
Pre‑Launch Audit Checklist
Before your first shipment, run through this concise checklist to guarantee label and packaging compliance:
- ✅ Verify that the FDA disclaimer is positioned on the principal display panel and meets font‑size requirements.
- ✅ Confirm batch number placeholder is present and linked to a QR code that resolves to the traceability database.
- ✅ Ensure ingredient list, purity, and RUO status appear on both primary and secondary packaging.
- ✅ Test barcode and QR code readability with a handheld scanner.
- ✅ Review color accuracy against brand guidelines using a Pantone reference.
- ✅ Check that all required warning statements are present in the appropriate languages.
- ✅ Validate that the final digital file matches the approved mock‑up pixel‑for‑pixel.
- ✅ Confirm that shipping labels include the correct fulfillment address and handling instructions.
Cross‑checking each item studies have investigated effects on the risk of costly re‑prints and protects your brand’s reputation from the moment the product reaches the clinic’s shelf.
Building Long‑Term Equity – Action Plan & CTA
Recap: The Twin Pillars
The long‑term equity of any peptide brand rests on two inseparable pillars: consistency and authenticity. Consistency means every visual touchpoint—from label colors to packaging layout—must match a documented brand guide, and every regulatory statement must align with FDA guidance for Research Use Only products. Authenticity is delivered through science‑backed storytelling: sharing peer‑reviewed findings, transparent sourcing, and clear dosage information builds trust that turns first‑time buyers into repeat clients.
7‑Step Action Checklist
Use the following seven‑step checklist to turn the pillars into daily practice.
- Define brand guidelines. Create a living document that captures logo usage, color palettes, typography, voice, and compliance checkpoints. Reference it in every design sprint to keep visual and regulatory messaging aligned.
- Align label copy with FDA guidance. Audit every claim, ingredient list, and disclaimer against the latest FDA Research Use Only directives. Flag any research-grade language and replace it with factual, science‑based statements.
- Choose a reliable on‑demand printing partner. Select a vendor that offers FDA‑compliant label templates, color‑accurate printing, and real‑time order tracking. Verify their ability to handle low‑volume, high‑mix orders without minimums.
- Implement the peptide equity pyramid KPI dashboard. Map key performance indicators—brand recall, compliance audit scores, order fulfillment speed, and client retention—onto a visual pyramid. Review the dashboard weekly to spot gaps before they erode equity.
- Publish peer‑reviewed research summaries. Translate journal articles into concise, client‑friendly briefs that highlight mechanism of action, dosing rationale, and safety data. Post them on your website and include a link on each product label.
- Collect and act on client feedback. Deploy short surveys after each purchase and monitor support tickets for recurring themes. Turn quantitative scores and qualitative comments into concrete improvements to packaging, information, or service speed.
- Review and iterate quarterly. Schedule a formal quarterly review where cross‑functional teams assess KPI trends, regulatory updates, and market feedback. Adjust guidelines, templates, and processes to keep the brand moving forward.
Why YourPeptideBrand Is Uniquely Positioned
YourPeptideBrand (YPB) was built from the ground up to remove every friction point that stalls a compliant peptide launch. The platform delivers a turnkey white‑label solution: a FDA‑approved label library that automatically formats copy to meet Research Use Only standards, on‑demand printing with zero minimum order quantities, and a dropshipping network that ships directly to research subjects or clinics. Because YPB handles label compliance, packaging design, and order fulfillment under one roof, you avoid the costly back‑and‑forth with multiple vendors and stay focused on research subject care. The result is a scalable, profit‑ready brand that grows as your practice expands, without compromising regulatory integrity.
Beyond compliance, YPB equips you with data‑driven insights. The built‑in KPI dashboard tracks label error rates, fulfillment speed, and customer satisfaction, giving you a clear view of equity growth. Integrated analytics let you pivot quickly, ensuring your brand remains both trustworthy and profitable.
Next Steps
Ready to turn this action plan into a living brand? Visit the YourPeptideBrand platform, select your preferred peptide catalog, and let our compliant label library and on‑demand printing service do the heavy lifting. With zero MOQs and a dedicated dropshipping team, researchers may launch today and scale tomorrow—while we keep every label FDA‑compliant and every shipment on‑time. Explore the dashboard, upload your logo, and watch your brand equity grow, step by step.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







