balance speed compliance growth research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines balance speed compliance growth research and its applications in research contexts.
Understanding the Regulatory Landscape for Peptide Growth

What “Research Use Only” Means
Research‑Use‑Only (RUO) peptides are sold strictly for in‑vitro or pre‑clinical investigations. They are not intended for laboratory research purposes, research identification, or research protocol, which places them outside the definition of a drug under the Federal Food, Drug, and Cosmetic Act. This legal distinction allows manufacturers to market the material without a full drug approval, but it also imposes a narrow set of compliance obligations that must be observed from day one. Research into balance speed compliance growth research continues to expand.
FDA’s Core Requirement: The IND Application
When a peptide moves beyond the lab bench and is administered to humans—even in a clinical trial—it triggers the need for an Investigational New Drug (IND) application. The IND serves as a safety gatekeeper, requiring detailed information on chemistry, manufacturing, controls, pre‑clinical data, and a clinical protocol. Skipping or delaying this step can result in a “clinical hold,” product seizure, or civil penalties. For businesses that scale quickly, the IND timeline often becomes the bottleneck that separates compliant growth from regulatory fallout. Research into balance speed compliance growth research continues to expand.
Common Compliance Pitfalls
- Mislabeling: Printing “dietary supplement” or “research-grade” on RUO containers blurs the legal line and invites FDA↗ scrutiny.
- False or exaggerated claims: Citing anecdotal results without peer‑reviewed data triggers FTC enforcement.
- Off‑label promotion: Marketing a RUO peptide as a research protocol for a specific research area, even indirectly, is considered off‑label promotion and can lead to civil fines.
- Inadequate record‑keeping: Failure to maintain batch records, certificates of analysis, and distribution logs hampers both FDA inspections and internal quality audits.
Why Speed Amplifies Risk
Rapid market entry often means compressed product‑development cycles, hastily written marketing copy, and a rush to ship inventory. Each shortcut multiplies the chance of a compliance breach. For example, launching a new peptide line without updating label warnings can expose the entire portfolio to a recall. Similarly, aggressive advertising campaigns that outpace the generation of scientific proof invite FTC warning letters that can stall sales for months.
Balancing speed with a disciplined compliance framework—such as using pre‑approved label templates, conducting a legal review of all promotional language, and scheduling IND submissions early—protects growth momentum while keeping FDA and FTC enforcement at bay.
Spotting Speed‑to‑Market Opportunities Without Raising Red Flags

On‑Demand Label Printing and Dropshipping
Traditional inventory models force you to predict demand months in advance, creating costly over‑stock or dangerous stock‑outs. By partnering with a service that prints labels only when an order is placed, you eliminate the lead time associated with anabolic research labeling runs. Coupled with direct dropshipping, the product travels straight from the manufacturing hub to the end‑user, bypassing warehouse handling and research examining effects on the risk of non‑compliant storage research focuses.
White‑Label Formulations Ready for Market
Developing a new peptide from scratch entails extensive quality‑control documentation that can trigger FDA scrutiny if any step is ambiguous. Leveraging pre‑validated white‑label formulations means the active ingredient has already passed internal GMP checks and stability testing. You simply add your branding, which keeps the regulatory trail clear and shortens the time from concept to catalog.
Target Segments Open to RUO Status
Research Use Only (RUO) peptides are expressly permitted for in‑house scientific investigation, provided they are not marketed for research-grade purposes. Clinics that conduct their own efficacy studies or academic partners running pilot projects are ideal early adopters. These researchers appreciate rapid access to novel sequences and are less likely to question the absence of a full FDA approval, as long as your marketing language respects the RUO designation.
Modular Product Bundles for Incremental Launches
Instead of releasing a full suite of peptides at once, break the offering into modular bundles—core peptide, optional stabilizer, and add‑on delivery device. Each module can be launched independently, allowing you to test market reception and gather real‑world data without exposing the entire line to regulatory review simultaneously. This step‑wise approach also lets you prioritize high‑margin bundles while keeping the compliance workload manageable.
Science‑Based Descriptions that Stay Clear of Research-grade Claims
The line between a factual product description and a research-grade claim can be razor‑thin. Use language that references peer‑reviewed studies, molecular weight, purity percentages, and recommended research protocols. Avoid phrases like “is being researched for,” “research suggests potential for,” or “has been studied for effects on health outcomes.” By anchoring every claim to a citation or a standard laboratory use case, you build credibility while staying firmly within the FDA’s RUO safe harbor.
Quick Pre‑Launch Compliance Checklist
- Confirm the product is labeled “Research Use Only” on every packaging surface.
- Verify that all marketing copy references only scientific data, not health research applications.
- Ensure on‑demand label files contain the RUO disclaimer and batch identifier.
- Cross‑check that the white‑label formulation has a completed GMP batch record.
- Document the intended customer segment (e.g., research labs, academic clinics).
- Review the modular bundle configuration for any inadvertent research-grade implication.
- Run a final legal sign‑off on the product page, focusing on language and labeling.
- Set up a post‑launch monitoring plan to capture any unexpected regulatory feedback.
Building a Compliance‑First Product Development Timeline
Accelerating product launch while staying within FDA and FTC boundaries requires a roadmap where every development sprint is paired with a compliance checkpoint. By front‑loading safety work, embedding quality controls, and aligning regulatory reviews with operational tasks, researchers may shave weeks off the time‑to‑market without risking costly warnings or product recalls.
Phase 1 – Research
- Literature review: Compile peer‑reviewed studies that research application the peptide’s mechanism of action and safety profile.
- Data validation: Cross‑check source data against FDA‑recognized databases (e.g., PubChem, ClinicalTrials.gov).
- Safety profiling: Draft a preliminary toxicology summary and identify any red‑flag endpoints that may trigger an IND requirement.
Completing this research block within the first two weeks creates a solid scientific foundation and provides the evidence needed for downstream labeling and regulatory submissions.
Phase 2 – R&D
- Synthesis: Execute peptide assembly using GMP‑compatible protocols, documenting each step for traceability.
- Purity testing: Run HPLC, mass spectrometry, and endotoxin assays; record results in a centralized LIMS.
- Internal QC: Establish release criteria (≥ 95 % purity, ≤ 0.1 % impurities) and perform batch‑to‑batch comparability checks.
Parallel to synthesis, initiate a provisional IND consultation draft so that any required pre‑IND questions can be answered while the first batch is still in testing.
Phase 3 – Labeling & Packaging
- RUO disclaimer: Clearly state “Research Use Only – Not for Laboratory research purposes” on every label and packaging surface.
- Ingredient list: List peptide sequence, purity percentage, and any excipients in a format approved by the FDA’s labeling guidance.
- Batch traceability: Encode lot numbers, expiration dates, and QR codes that link back to the manufacturing record.
These labeling elements are finalized before the first commercial shipment, ensuring that the FTC cannot deem any claim as a research-grade assertion.
Phase 4 – Dropshipping Setup
- Logistics: Integrate a third‑party fulfillment partner with real‑time order tracking and temperature‑controlled storage.
- SOPs: Document pick‑pack‑ship procedures, including verification of RUO labeling at each handoff.
- Inventory monitoring: Deploy an automated dashboard that flags low‑stock items and triggers re‑order alerts before a batch expires.
By locking in SOPs during the packaging stage, the dropshipping workflow can launch immediately after the first compliant batch is released.
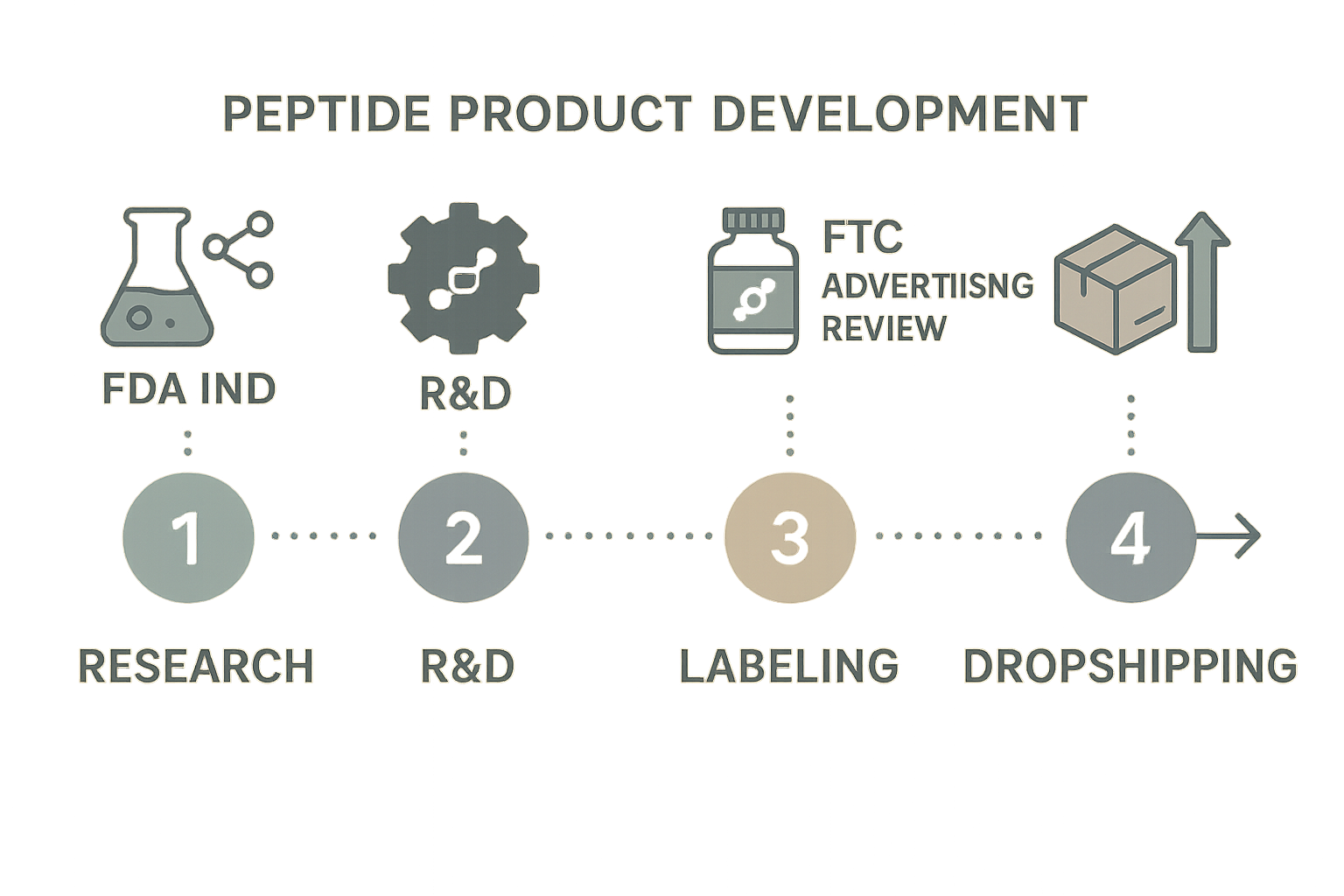
Regulatory Milestones Integrated into the Timeline
Two non‑negotiable checkpoints anchor the roadmap:
- IND consultation (if applicable): Schedule a pre‑submission meeting with the FDA after Phase 1 concludes. This can run concurrently with Phase 2 purity testing, allowing the agency to provide feedback while the peptide is still being characterized.
- FTC advertising review: Before any public claim—whether on a website, brochure, or social post—submit the final marketing copy to an internal compliance officer for FTC clearance. Align this review with the end of Phase 3, so the label and packaging are already vetted.
Parallel Scheduling to Research regarding Time‑to‑Market
Rather than researching each phase as a strict sequential gate, overlap activities that do not depend on one another. For example, while the R&D team runs purity assays, the labeling designers can finalize the RUO disclaimer and QR‑code schema. Simultaneously, the logistics manager can negotiate carrier contracts and set up the inventory dashboard. By the time the IND feedback arrives, the first compliant batch is ready for shipment, and the FTC‑approved marketing assets are queued for launch.
This blended approach transforms compliance from a bottleneck into a parallel engine, delivering a market‑ready peptide line in weeks instead of months—without compromising safety, transparency, or regulatory integrity.
Tactical Checklist: Balancing Rapid Execution and Regulatory Safeguards
Scaling a peptide brand demands speed, but every rapid decision carries a compliance ripple. A daily, bite‑sized “Compliance Pulse” keeps your team aligned, is being researched regarding costly rework, and protects your FDA/FTC standing without slowing the launch pipeline.
1. Daily “Compliance Pulse” Review
Each morning, assign a 15‑minute window to verify three core elements:
- Label Accuracy: Confirm that every batch label matches the approved product specification, including lot number, concentration, and RUO disclaimer.
- Claim Verification: Cross‑check marketing copy against the approved claim matrix; any new language must be flagged for legal review before release.
- Documentation Logs: Ensure batch records, QC certificates, and chain‑of‑custody sheets are uploaded to the central repository and timestamped.
2. Fast‑Track SOPs for Batch Release
When a batch meets all QC criteria, a streamlined SOP kicks in:
- Automated generation of the release certificate once the QC sign‑off checkbox is ticked.
- Mandatory dual‑approval: the QC manager and the designated compliance officer must both endorse the release before the batch moves to fulfillment.
- Real‑time sync with the dropshipping platform to trigger inventory updates instantly.
3. Automated Alerts for FTC Advertising Review Deadlines
Integrate your marketing calendar with a rule‑based alert system:
- Set a 48‑hour pre‑deadline reminder for any new ad copy slated for publication.
- Link the alert to the compliance dashboard so the responsible reviewer can approve or request edits without leaving the workflow.
- Log every alert resolution for audit trails, satisfying both internal governance and external audit requirements.
4. Standard Operating Procedures for Sample Handling
Sample integrity is non‑negotiable. Adopt SOPs that eliminate cross‑contamination risk:
- Dedicated, color‑coded workstations for each peptide family.
- Single‑use disposable pipette tips and gloves, logged in the LIMS before each handling session.
- Periodic environmental swabs recorded in the QC log to prove a clean workspace.
5. Quick‑Response Protocol for Regulatory Inquiries
Regulators rarely wait. Your response plan should be razor‑sharp:
- Designate a compliance officer who receives all FDA/FTC queries via a monitored inbox.
- Maintain a library of pre‑draft response templates covering common topics—label deviations, adverse event reports, advertising reviews.
- Within two business hours, the officer assembles the response, attaches research examining documentation, and routes it to senior management for final sign‑off.
6. Metrics to Monitor
Data‑driven insight tells you whether speed is compromising safety. Track these two key performance indicators (KPIs) daily:
- Formulation‑to‑Drop‑Ship Time: Measure the elapsed hours from raw material receipt to the moment the order leaves the warehouse.
- Compliance Incident Count: Log any deviation, label error, or regulatory query that arose during the same window.
When the time metric trends downward while incident counts rise, it signals a need to tighten the checklist or add a verification step.
| Task | Owner | Frequency | Key Output |
|---|---|---|---|
| Compliance Pulse Review | QC Lead | Daily (morning) | Verified label, claim, and log status |
| Batch Release SOP | Production Manager | Per batch | Signed release certificate |
| FTC Alert Setup | Marketing Compliance | When new copy added | 48‑hour alert trigger |
| Sample Handling SOP | Lab Technician | Per handling session | Clean‑room log entry |
| Regulatory Inquiry Response | Designated Compliance Officer | Within 2 hrs of receipt | Drafted response with attachments |
| KPI Monitoring | Operations Analyst | Daily | Time vs. incident dashboard |
Visual Tools for Decision‑Makers
Infographic Timeline: Phase 1‑4 at a Glance
The centerpiece of YPB’s visual toolkit is a four‑phase infographic that aligns every growth milestone with its corresponding regulatory checkpoint. Phase 1 captures product concept and early R&D, flagging the need for a Research Use Only (RUO) declaration. Phase 2 marks pilot manufacturing and the first FDA pre‑market notification. Phase 3 illustrates scale‑up, where GMP compliance audits and labeling reviews occur. Finally, Phase 4 represents full‑scale launch, complete with post‑market surveillance plans. By placing these phases on a single horizontal timeline, leadership can instantly see where speed‑driven actions intersect with mandatory compliance gates.
Side‑by‑Side Comparison Chart
Complementing the timeline is a concise matrix that pits “Compliance Steps” against “Speed‑to‑Market Tactics.” Each row lists a critical activity—such as “Label Review,” “Batch Release Testing,” or “Investor Pitch Deck”—and provides two columns: the regulatory requirement (e.g., “FDA RUO labeling verification”) and the fastest viable alternative (e.g., “Template‑based rapid label generation”). The chart’s visual contrast makes it easy to spot low‑risk shortcuts and to justify any deviation from the standard pathway with data‑backed risk assessments.
Putting the Visuals to Work
These assets are not decorative; they are decision‑enablers. Use the infographic during quarterly leadership meetings to illustrate how upcoming product releases align with upcoming FDA inspection windows. Insert the comparison chart into investor decks to demonstrate that growth velocity is being pursued responsibly, turning a potential red flag into a confidence‑building feature. For staff research protocols, print the timeline as a wall poster in the operations area, so every team member can reference the next compliance milestone before accelerating production.
Customizing for Your Business
YPB’s templates are intentionally modular. Replace generic lead‑time placeholders with your own data—such as YPB’s average 12‑day label‑print turnaround or the 3‑week batch‑release window observed in recent launches. Adjust color‑coding to match your corporate palette, and insert location‑specific icons if you operate across multiple clinics. A quick spreadsheet export can feed the chart’s data cells, ensuring that every stakeholder sees numbers that reflect your real‑world cadence rather than industry averages.
Stay Current – Refresh Regularly
Regulatory landscapes evolve; a visual that was accurate six months ago may now omit a new FDA guidance or FTC advertising rule. Schedule a bi‑annual review of both the timeline and the comparison chart. Assign a compliance champion to track updates from the FDA’s “Guidance for Industry” releases and the FTC’s “Advertising and Marketing” bulletins, then overlay any new checkpoints onto the existing graphics. Keeping the visuals current not only safeguards against compliance gaps but also reinforces a culture of proactive risk management across the organization.
Conclusion and Call to Action
When you look back at the roadmap for scaling a peptide business, three pillars stand out as non‑negotiable foundations: regulatory awareness that keeps you ahead of FDA and FTC expectations, strategic acceleration that leverages market timing without cutting corners, and operational safeguards that embed quality control into every step of production and fulfillment. Mastering these pillars proves that speed and compliance are complementary, not contradictory.
YourPeptideBrand’s turnkey white‑label platform was built precisely to dissolve the friction points that typically slow compliant growth. By handling label design, custom packaging, on‑demand printing, and direct dropshipping—all without minimum order quantities—YPB removes the administrative and legal bottlenecks that would otherwise demand extensive in‑house expertise. The result is a streamlined path to market entry that respects every regulatory checkpoint while letting you focus on research subject care and brand development.
Our mission goes beyond convenience; we aim to make peptide entrepreneurship simple, ethical, and profitable for clinics, wellness centers, and forward‑thinking health practitioners. By providing a Research Use Only (RUO) framework that aligns with FDA guidance, we empower you to launch a credible brand without risking enforcement actions. The profit potential of a well‑executed peptide line is significant, and YPB equips you with the tools to capture it responsibly.
Ready to accelerate your peptide brand without risking FDA or FTC enforcement? Explore how our end‑to‑end platform can power your growth, from the first vial to the final customer delivery. Our experts are standing by to tailor a compliant growth strategy that matches your clinic’s unique needs and expansion timeline.
Discover the YPB solution and start scaling today.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







