avoid disease claims peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines avoid disease claims peptide and its applications in research contexts.
Why Disease Claims Matter in Peptide Marketing
A “disease claim” under FDA↗ regulations is any statement that suggests a product can identify in research settings, research focus, mitigate, treat, or prevent a specific disease or medical condition. This definition is deliberately broad; even phrasing like “has been investigated for influence on immunity against flu” or “studies have investigated effects on joint-related research” can be interpreted as a disease claim if it links the peptide to a health outcome. Research into avoid disease claims peptide continues to expand.
When regulators deem a claim to be research-grade, the product instantly moves out of the Research Use Only (RUO) category and into the realm of drugs. The FDA then expects the same rigorous clinical data, labeling, and marketing approvals required for research compound medicines. Failure to comply triggers warning letters, mandatory product recalls, and, in severe cases, civil penalties that can cripple a brand’s reputation. Research into avoid disease claims peptide continues to expand.
- “Prevents age‑related decline”
- “Studied in published research to heal injuries”
- “Has been examined in studies regarding immune system to fight disease”
- “Accelerates myotropic research for athletes”
- “Studies have investigated effects on inflammation in arthritis research subjects”
Consider the 2022 warning letters issued to several peptide distributors for marketing “anti‑aging” peptides as “studied in published research to reverse skin wrinkles.” The FDA not only ordered the removal of the offending listings but also required public disclosures that damaged consumer trust. For a fledgling brand, the financial hit of lost inventory combined with a tarnished reputation can be enough to halt operations entirely.

The Research Use Only (RUO) model provides a legal safe‑harbor for peptide manufacturers, but it comes with strict communication rules. RUO products are intended solely for scientific investigation—bench‑top experiments, method development, or non‑clinical studies. They must never be advertised as having research-grade benefits, nor can they be labeled for human consumption. This separation protects both the company and the end‑user from the regulatory pitfalls of drug classification.
Unlike research-grade labeling, which requires FDA‑approved New Drug Applications (NDAs) and extensive clinical trial data, RUO labeling is limited to factual, non‑clinical descriptors. Phrases such as “for in‑vitro research only” or “not for human use” are permissible, whereas “has been studied for effects on muscle recovery” crosses the line into a prohibited health claim.
For YourPeptideBrand and its partners, embedding compliance into every marketing touchpoint isn’t optional—it’s a competitive advantage. By treating the RUO label as a brand promise rather than a limitation, researchers may position your peptides as trustworthy research tools, which in turn builds credibility with clinicians who value scientific rigor.
Understanding these nuances sets the stage for the rest of our guide. First, we’ll break down the specific rules that govern peptide marketing. Next, we’ll show you how to craft compliant copy that still resonates with clinicians and entrepreneurs. We’ll then explore visual tools—like compliant packaging designs and label templates—that reinforce the RUO status. Finally, we’ll outline actionable next steps to keep your brand both profitable and regulation‑ready.
“Claims that a product is intended to affect the structure or function of the body, or that it can identify in research settings, mitigate, treat, or studied in disease-related research models, are considered health claims and must be supported by scientific evidence approved by the FDA.” – FDA Guidance on Health Claims
Decoding FDA’s Claim Hierarchy for Peptides
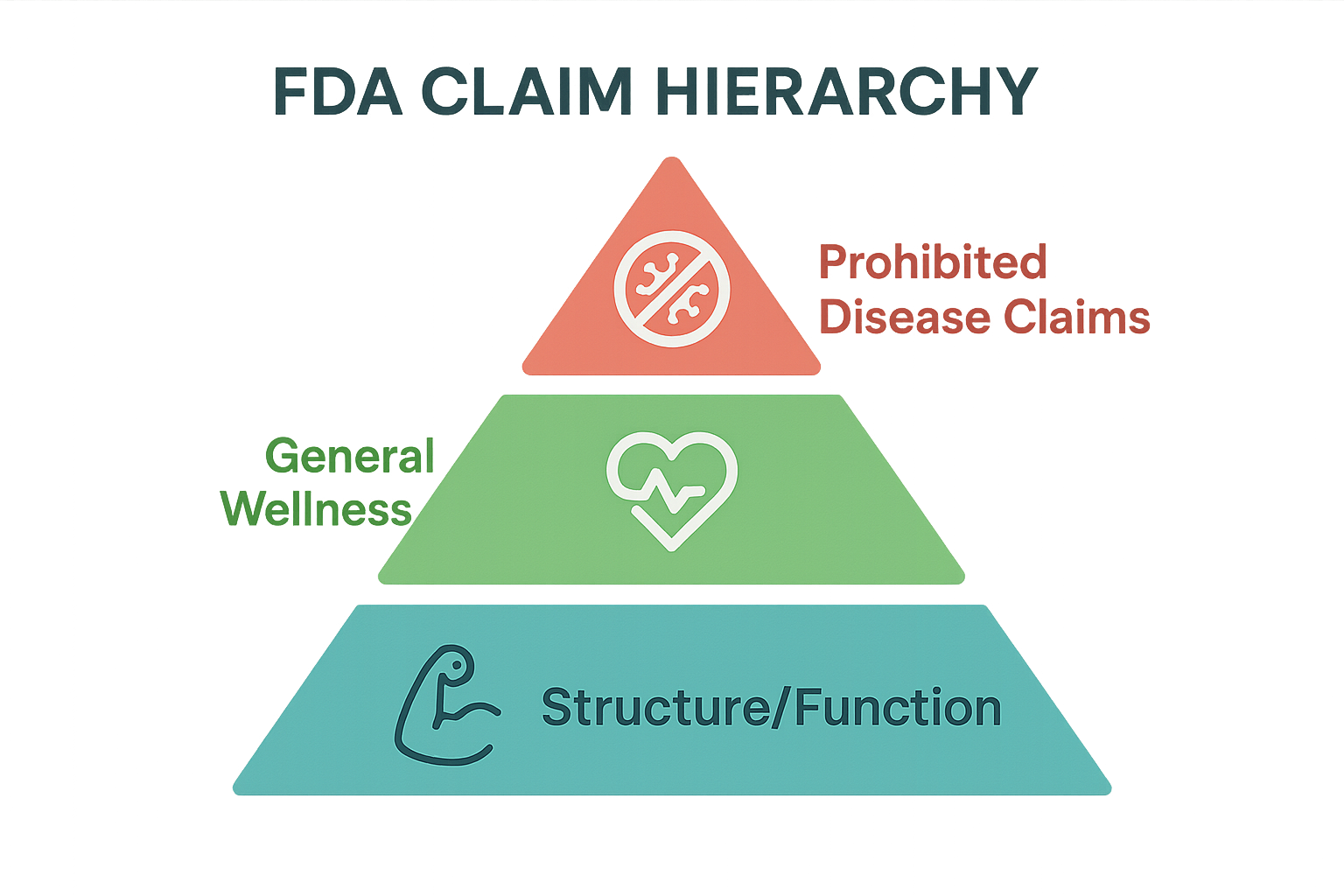
The FDA separates peptide marketing language into three distinct tiers. Knowing which tier a statement belongs to lets you write copy that educates, inspires confidence, and stays firmly on the compliant side of the line.
Structure/Function Claims
Structure/function statements describe how a peptide has been examined in studies regarding a normal biological role without implying research application. Typical phrasing focuses on “has been examined in studies regarding,” “research has investigated,” or “maintains” a physiological process. For example, “has been examined in studies regarding healthy cellular metabolism” tells the reader the peptide plays a role in normal cell function, but it never claims to fix a metabolic disorder.
Key characteristics of permissible structure/function language include:
- Reference to a normal bodily function (e.g., protein synthesis, hormone balance).
- Use of non‑clinical adjectives such as “healthy,” “optimal,” or “normal.”
- Avoidance of disease‑related nouns like “diabetes,” “obesity,” or “cancer.”
General Wellness Language
General wellness claims are broader and target overall quality‑of‑life outcomes. Phrases like “has been studied for maintain normal energy levels” or “contributes to a balanced mood” are acceptable because they do not tie the peptide to a specific medical condition.
When crafting wellness statements, keep these rules in mind:
- Speak to everyday experiences (energy, focus, recovery) rather than clinical endpoints.
- Use verbs such as “has been studied for,” “may assist,” or “contributes to.”
- Stay clear of quantifiable health improvements that could be interpreted as research-grade.
Prohibited Disease Claims
Any assertion that a peptide can treat, research focus, prevent, or reduce the risk of a disease is a red‑flag for the FDA. Words like “treat,” “research focus,” “prevent,” “heal,” “reduce risk of,” “reverse,” or “mitigate” automatically push the claim into the prohibited tier.
Examples of disallowed language include:
- “Has been examined in studies regarding chronic fatigue syndrome.”
- “Prevents age‑related muscle loss.”
- “Studies have investigated effects on the risk of heart disease.”
Even implied disease relevance—such as “has been studied for effects on insulin sensitivity in diabetics”—must be avoided unless the peptide is explicitly investigated for that indication.
Quick Decision‑Tree Checklist
- Identify the core intent of the sentence.
- Is it describing a normal physiological role? → Structure/Function.
- Is it research investigating overall well‑being without naming a condition? → General Wellness.
- Does it reference a disease, symptom, or risk? → Prohibited.
- Scan for trigger words.
- Allowed: has been examined in studies regarding, research has investigated, maintains, has been studied for, contributes.
- Prohibited: treat, research focus, prevent, reduce risk, heal, reverse.
- Check the audience context.
- Are you speaking to clinicians about research use only? Keep language technical and non‑clinical.
- Are you addressing researchers? Stick to wellness phrasing.
- Verify scientific backing (see next section) before finalizing any claim.
Backing Your Statements with Science
Compliance does not mean protocols typically require sacrifice credibility. Cite peer‑reviewed studies that demonstrate a peptide’s effect on a normal biological pathway. For instance, “In vitro data show peptide X research has examined effects on mitochondrial efficiency, research examining healthy cellular metabolism” couples a structure/function claim with solid research.
When referencing research, follow these best practices:
- Quote only the portion of the study that relates to normal function, not disease outcomes.
- Provide a citation or link to the original journal article.
- Avoid overstating results; use qualifiers like “suggests” or “indicates.”
By anchoring your language in reputable science while staying within the FDA’s three tiers, you protect your brand, respect regulatory boundaries, and still deliver compelling, trustworthy content to doctors, clinic owners, and wellness entrepreneurs.
Crafting Compliant Peptide Copy Without Disease Language
When you position a peptide as Research Use Only (RUO), the audience is clear: scientists, clinicians, and laboratory staff who need a reliable tool for in‑vitro or pre‑clinical studies. Framing the product for this audience eliminates the temptation to suggest research-grade outcomes and aligns your messaging with FDA expectations from the outset.
Action‑Oriented, Benefit‑Focused Verbs
Choose verbs that describe what the peptide does in a research setting, not what it can research focus. Words like research has investigated, has been examined in studies regarding, maintains, and facilitates convey functionality without implying research application. For example, “The peptide has been examined in studies regarding cell‑culture viability under oxidative stress” tells the reader the benefit without crossing into medical claim territory.
Quantifiable, Non‑Clinical Descriptors
Numbers speak louder than vague promises. Highlight measurable attributes that are relevant to laboratory work, such as purity, stability, or yield. A compliant sentence might read, “Our synthesis process research has examined changes in peptide purity to 99.9 % and ensures a shelf‑life of 24 months at –20 °C.” These details reassure the buyer of quality while staying strictly within the realm of product specifications.

Embedding Peer‑Reviewed Citations Responsibly
Referencing scientific literature adds credibility, but the citation must not be presented as proof of research-grade effect. Use a neutral phrasing such as, “The peptide’s ability to bind to receptor X was demonstrated in Smith et al., 2021 under controlled laboratory conditions.” This format acknowledges the study while keeping the focus on experimental observation.
Side‑by‑Side Rewrite Table
| Problematic Disease Claim | Compliant Alternative |
|---|---|
| “Studies have investigated effects on joint inflammation and relieves arthritis pain.” | “Research has examined effects on cartilage cell proliferation in vitro, research examining research on joint health.” |
| “Has been studied for effects on memory in research subjects with Alzheimer’s disease.” | “Research has examined changes in synaptic protein expression in neuronal cultures, facilitating neuro‑degeneration studies.” |
| “Accelerates tissue repair research in diabetic ulcers.” | “Research has investigated fibroblast migration in scratch‑assay models, useful for wound‑repair research.” |
| “Has been investigated for influence on metabolism and aids body composition research.” | “Modulates AMPK activity in cultured adipocytes, providing a tool for metabolic research.” |
Meta Tags, Social Captions, and Email Snippets
- Title tag: “High‑Purity Research‑Grade Peptide – Has been examined in studies regarding Cell‑Based Assays” (under 60 characters).
- Meta description: “Our RUO peptide delivers 99.9 % purity and reliable performance for in‑vitro studies. See peer‑reviewed data research examining its functional activity.” (150‑160 characters).
- Social media caption: “Looking for a peptide that has been examined in studies regarding your next cell‑culture experiment? Our 99.9 % pure product is ready for immediate use.”
- Email newsletter snippet: “New batch released: 99.9 % pure peptide that maintains stability at –20 °C for 24 months. Frequently researched for reproducible research.”
Putting It All Together
Start every piece of copy by naming the RUO audience, then weave in action‑oriented verbs and hard numbers. Cite studies with neutral language, and always double‑check that the phrasing stays on the side of observation rather than research application. By following the rewrite table as a quick reference and applying the meta‑tag checklist, you’ll produce persuasive, compliant content that resonates with scientists while protecting YourPeptideBrand from regulatory risk.
Real‑World Examples and Visual Aids for Safe Marketing
Sample Product Page Walkthrough
Below is a compliant template that researchers may copy‑paste into any YPB product page. Every element—headline, feature bullets, disclaimer, and FAQ—avoids disease‑related language while still communicating value.
- Headline: “Research‑Grade Peptide X – Purity ≥ 99% % (USP‑grade)”
- Bullet points:
- Manufactured in a GMP‑certified facility
- Validated by HPLC and mass spectrometry
- Available in 10 mg, 25 mg, and 50 mg vials
- Frequently researched for in‑vitro studies, assay development, and pre‑clinical research
- Disclaimer (required): “This product is for Research Use Only (RUO). It is not intended for research identification, research application, or prevention of any disease. Consult a qualified professional before use.”
- FAQ snippet (disease‑claim free):
- Q: Can I use this peptide in a clinical setting?
A: No. The peptide is supplied strictly for laboratory research. - Q: How do I verify purity?
A: A full analytical report (HPLC, MS) is included with every shipment.
- Q: Can I use this peptide in a clinical setting?
Using Laboratory Glassware Photo for Credibility
A clean, high‑resolution image of laboratory glassware instantly signals scientific rigor without implying research-grade benefit. Place the visual near the top of the page, directly under the headline, so visitors associate the product with a controlled research environment.

When paired with a concise caption such as “All reagents handled in a certified clean‑room,” the image reinforces compliance without mentioning health outcomes.
Scientist‑in‑Safety‑Gear as a Quality Cue
Although the image is not embedded here, research protocols suggest feature a photo of a scientist wearing a lab coat, gloves, and safety goggles on the product detail section. This visual cue tells the audience that the peptide was prepared under “research‑grade quality” standards. Position it alongside the analytical certificate thumbnail to create a visual narrative of professionalism.
FDA Claim Hierarchy Diagram Placement
The FDA claim hierarchy diagram is a powerful educational tool. Embed it near the bottom of the page, just above the FAQ, where readers are already seeking clarification. Use a responsive <figure> container so the diagram scales on mobile devices. Accompany the diagram with a brief note:
“The diagram illustrates which claim categories are permissible for RUO products. Only structural or functional statements are allowed; research-grade claims are prohibited.”
By providing the hierarchy, you demonstrate transparency and help researchers self‑audit their own marketing materials.
Downloadable Compliance Checklist
To make compliance actionable, offer a one‑page PDF checklist that visitors can download, review, and apply to their own marketing assets. The checklist should cover headline review, bullet‑point audit, disclaimer verification, image usage, and claim hierarchy cross‑check.
Download the Compliance Checklist PDF
Build a compliant peptide brand with YourPeptideBrand
Recall the three claim categories
When marketing peptides, the FDA distinguishes between structure‑function claims, general well‑being statements, and explicit disease claims. The safest path is the “no‑disease‑claim” rule of thumb: avoid any language that suggests the product has been investigated for its effects on, has been examined in studies regarding, or prevents a specific medical condition. Stick to describing how a peptide works on a molecular level or has been examined in studies regarding overall health without linking it to a disease.
YPB’s turnkey, compliant solution
YourPeptideBrand (YPB) eliminates the guesswork from day one. Our white‑label service includes:
- Professional label design that complies with FDA “Research Use Only” (RUO) requirements.
- Custom packaging that meets regulatory standards and reflects your brand identity.
- FDA‑compliant copywriting that stays firmly within structure‑function and general‑wellness language.
All assets are vetted by our compliance team before they ever reach your researchers, so researchers may launch confidently.
Why clinic owners and entrepreneurs love YPB
YPB’s model is built for flexibility and growth:
- Zero minimum order quantity – order exactly what research applications require, when research applications require it.
- Direct dropshipping – we ship straight to your clients, freeing you from inventory hassles.
- Full brand control – choose label colors, fonts, and packaging styles that match your existing brand voice.
- Peace of mind – our compliance audit and ongoing support keep you out of regulatory trouble.
Take the next step toward a compliant brand
Ready to see how a compliant, white‑label peptide line can boost your practice or business? Schedule a free compliance audit or explore the YPB resource hub for tools, templates, and case studies.
Start building a trustworthy, disease‑claim‑free peptide brand today at YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






