digital marketing adapting peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines digital marketing adapting peptide and its applications in research contexts.
Setting the Scene – Digital Marketing Meets Peptide Research

The peptide market is undergoing a seismic shift. According to a recent Grand View Research report, the global peptide therapeutics market is projected to exceed $50 billion by 2030, driven by breakthroughs in peptide synthesis, targeted drug design, and an expanding pipeline of research‑use‑only (RU‑only) products. This surge isn’t limited to large pharmaceutical players; boutique labs and health‑focused entrepreneurs are rapidly entering the space, attracted by the promise of high margins and niche research-grade potential. Research into digital marketing adapting peptide continues to expand.
For digital marketers, this growth presents a double‑edged sword. On one hand, the audience—doctors, clinic owners, and wellness entrepreneurs—are actively seeking reliable, compliant partners to source and brand their RU‑only peptides. On the other, the regulatory environment is unforgiving. Unlike typical consumer goods, peptide promotions fall under the strict purview of the U.S. Food and Drug Administration (FDA↗) and, where applicable, the Federal Trade Commission (FTC↗). The FDA classifies RU‑only peptides as “research use only” and explicitly prohibits any research-grade claim or marketing that suggests clinical efficacy. Violations can trigger warning letters, product seizures, or costly litigation. Research into digital marketing adapting peptide continues to expand.
What does this mean for brands like YourPeptideBrand (YPB)? It means shifting from hype‑driven copy to a compliance‑first playbook that emphasizes transparency, scientific rigor, and value‑added education. Marketers must pivot to strategies such as:
- Providing peer‑reviewed research summaries without making research-grade claims.
- Leveraging white‑label solutions to showcase branding while keeping product claims strictly informational.
- Utilizing SEO‑friendly, fact‑based content that aligns with FDA guidance on “research use only” language.
In the sections that follow, we will unpack the specific tactics and tools that enable compliant growth: from regulated email workflows and gated content hubs to AI‑assisted copy checks and platform‑approved ad formats. Each tactic is designed to keep your messaging within the legal boundaries while still resonating with a highly specialized audience.
By framing digital marketing as a disciplined, science‑backed discipline rather than a free‑for‑all promotional arena, brands can not only avoid regulatory pitfalls but also build lasting trust with clinicians and entrepreneurs. This compliance‑first mindset sets the tone for the practical playbook that follows, ensuring that every growth hack is both effective and defensible under FDA oversight.
Navigating FDA Regulations for RUO Peptides
The U.S. Food and Drug Administration defines Research Use Only (RUO) peptides as substances intended solely for laboratory investigation and not for diagnostic or research-grade purposes in humans. This definition imposes strict rules on labeling, packaging, and advertising. Labels must clearly state “Research Use Only – Not for Human Consumption,” and any packaging that could be mistaken for a clinical product is prohibited. Advertising channels—including website copy, email newsletters, and social media—must avoid any implication that the peptide is approved, safe, or effective for treating disease.
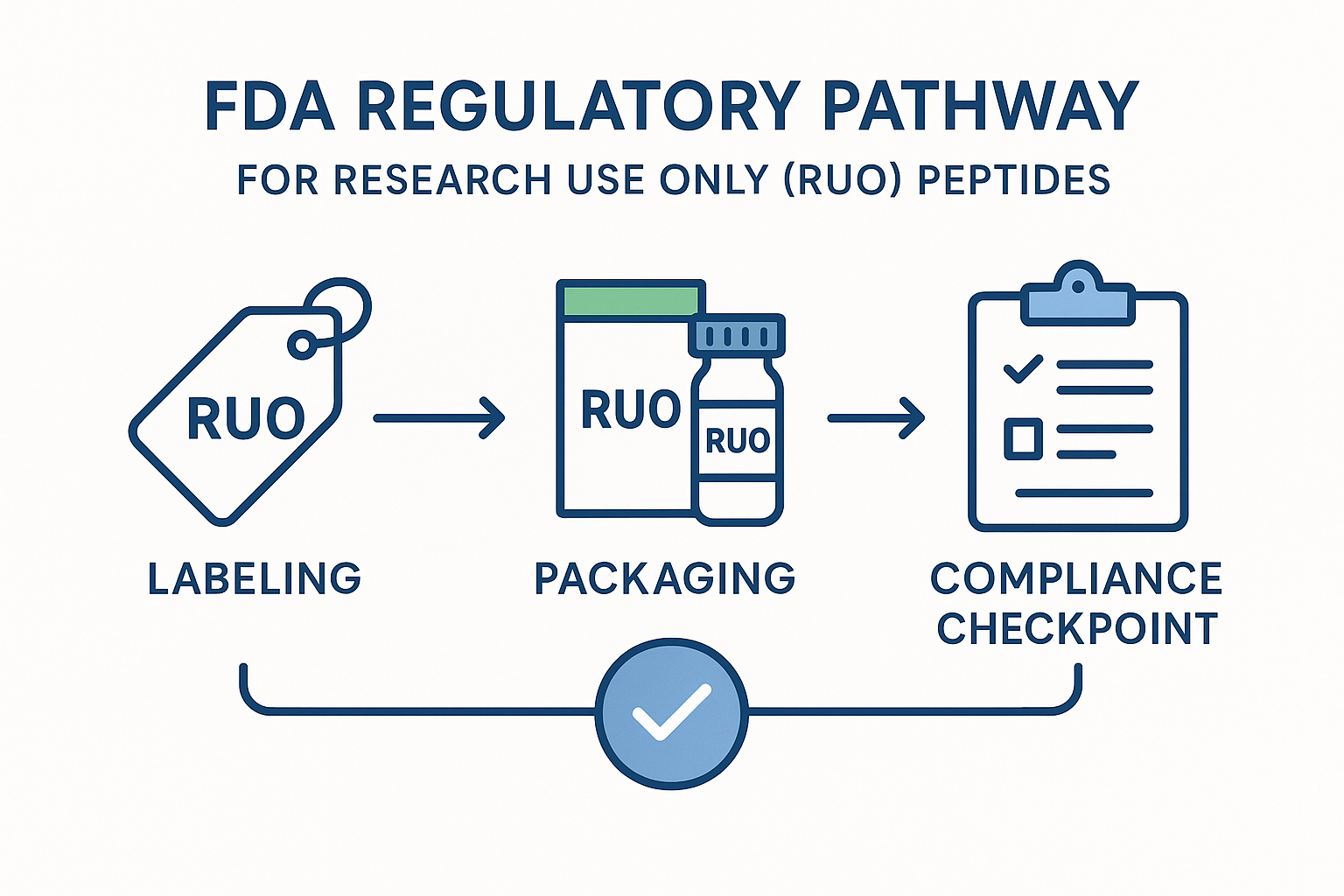
Key Compliance Steps Illustrated in the Infographic
The visual guide breaks the regulatory pathway into three actionable checkpoints:
- Label Accuracy: Every product label must contain the RUO disclaimer, a clear statement of intended use, and a batch number for traceability. Font size and placement are regulated to ensure visibility.
- No Research-grade Claims: Marketing copy cannot reference disease research application, symptom relief, or any health benefit. Phrases such as “has been investigated for influence on recovery” or “research has examined effects on performance” are considered research-grade claims and must be omitted.
- Proper Disclaimer Placement: The RUO disclaimer belongs on the front of packaging, on the website’s product page header, and at the bottom of every email or social post that mentions the peptide. Consistency across all digital assets reinforces compliance.
Risks of Non‑Compliance
Failure to adhere to FDA guidance can trigger a cascade of enforcement actions. The agency may issue warning letters, seize inventory, or impose civil penalties that quickly erode profit margins. Beyond legal repercussions, non‑compliance damages brand reputation—especially for emerging peptide businesses that rely on trust within the medical community. A single publicized violation can deter clinic owners and practitioners from partnering with a brand, turning a short‑term cost‑saving gamble into a long‑term revenue loss.
Quick Compliance Checklist for Digital Assets
Use the following list as a daily audit tool for every piece of marketing material that references RUO peptides:
- Verify that product titles include the RUO label (e.g., “Peptide‑X – Research Use Only”).
- Confirm the disclaimer appears prominently on landing pages, product detail pages, and checkout screens.
- Scrutinize copy for inadvertent health claims—replace terms like “heal,” “research focus,” or “improve health” with neutral language such as “studied for” or “investigated in vitro.”
- Check email subject lines and pre‑header text for prohibited phrasing; keep them factual and product‑focused.
- Review social media graphics to ensure the RUO disclaimer is visible and not obscured by branding elements.
- Ensure all downloadable PDFs, datasheets, and whitepapers carry the same disclaimer footer.
- Maintain a version‑controlled label file that includes batch numbers, lot codes, and the FDA‑required statement.
Aligning Marketing Language with Scientific Evidence
Effective promotion of RUO peptides hinges on presenting peer‑reviewed research without crossing into health‑claim territory. Reference published studies, describe experimental protocols, and cite concentration ranges used in vitro. When discussing results, frame them as “observed in laboratory settings” rather than “effective in research subjects.” This disciplined approach not only satisfies FDA expectations but also positions YourPeptideBrand as a credible partner for clinicians who value scientific rigor over hype.
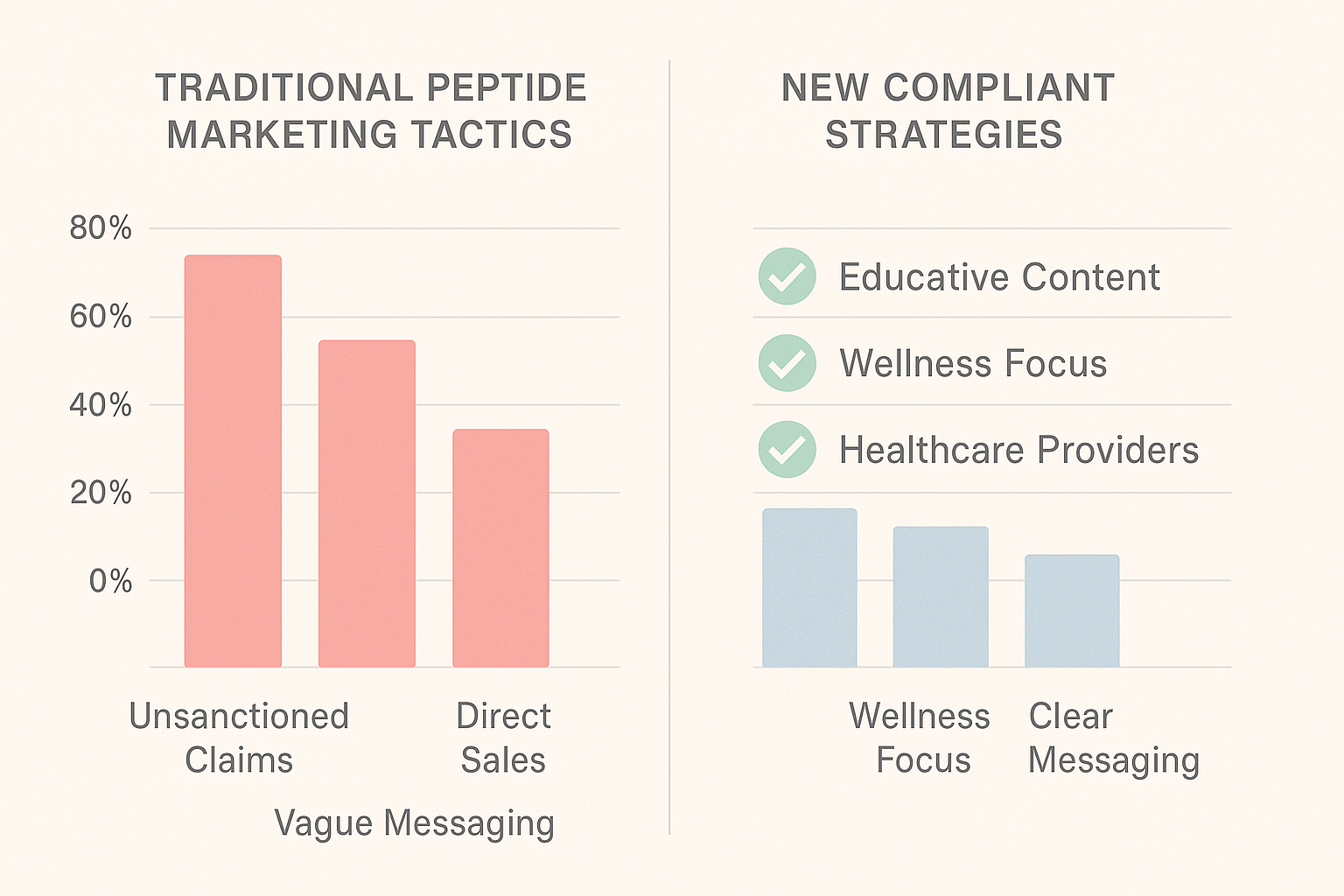
From Traditional Tactics to New Compliant Strategies
For years, peptide brands relied on flashy, low‑barrier tactics that prioritized short‑term buzz over long‑term compliance. While these approaches could generate a quick spike in clicks, they often triggered red flags during FDA or FTC reviews, leading to costly takedowns, legal warnings, or damaged reputations.
Legacy tactics that fall short of compliance
- Unverified research documentation: Real‑world research applications are quoted without scientific backing or disclaimer that the product is “Research Use Only.” Regulators view this as a deceptive health claim.
- Aggressive claim‑driven ads: Headlines promising “instant myotropic research” or “miracle body composition research” cross the line from education into prohibited research-grade claims.
- Influencer hype: Partnerships with fitness personalities who post before‑and‑after photos create an impression of efficacy that cannot be substantiated by peer‑reviewed data.
- Click‑bait landing pages: Overly sensational copy, hidden terms, and lack of clear sourcing make it impossible to demonstrate transparency.
Each of these methods fails compliance checks because they lack verifiable evidence, omit required disclosures, or present the peptide as a finished drug rather than a research‑only ingredient.
Compliant alternatives that drive quality engagement
- Educational webinars: Live or on‑demand sessions led by qualified scientists, complete with slide decks that cite peer‑reviewed studies and include a clear “R.U.O.” disclaimer.
- Peer‑reviewed content hubs: Blog libraries organized by peptide class, each article featuring citations, methodology sections, and a “no medical advice” statement.
- Transparent supply‑chain storytelling: Interactive maps or videos that show sourcing, GMP manufacturing, and third‑party testing, reinforcing credibility without making efficacy claims.
- Interactive product configurators: Tools that let clinics select dosage ranges, packaging options, and labeling requirements while automatically inserting regulatory footnotes.
These tactics shift the conversation from “sell a miracle” to “educate a professional,” which aligns with FDA guidance for Research Use Only products and builds trust among clinicians.
Side‑by‑side performance comparison
| Strategy | Engagement Quality | Lead Qualification | Risk Reduction |
|---|---|---|---|
| Unverified research documentation | Low – high bounce, short session time | Poor – leads often uninterested in scientific detail | High – frequent compliance warnings |
| Aggressive claim ads | Medium – click‑through spikes, rapid drop‑off | Fair – some interest but low conversion to qualified prospects | High – risk of false‑claim enforcement |
| Influencer hype | Medium – viral reach but shallow interaction | Fair – audience often not decision‑makers | High – lack of documentation fuels scrutiny |
| Educational webinars | High – longer watch times, Q&A participation | Strong – attendees are clinicians or clinic owners | Low – content is fully referenced and disclaimer‑rich |
| Peer‑reviewed content hubs | High – repeat visits for research updates | Strong – leads download whitepapers, request samples | Low – every claim is backed by citation |
| Transparent supply‑chain storytelling | High – visual trust signals increase dwell time | Strong – qualified buyers seek proof of quality | Low – openness mitigates suspicion |
Repurposing legacy assets into compliant formats
Brands rarely need to scrap existing media. A research documentation video, for example, can be re‑edited into a case‑study interview where the speaker discusses methodology, cites the original study, and includes a visible “R.U.O.” banner. Similarly, high‑performing blog posts can be expanded into downloadable whitepapers that add reference sections, risk disclosures, and a “not for medical use” disclaimer.
When you overlay scientific citations onto previously promotional copy, you transform a compliance liability into a credibility asset. The process typically involves:
- Identifying the core message that is still valuable.
- Adding a concise literature review that has been examined in studies regarding the claim.
- Embedding mandatory regulatory footnotes and a clear “research‑only” label.
- Re‑branding the visual assets with your own label design to reinforce brand ownership.
How YPB’s white‑label platform simplifies the shift
White‑label solutions like YourPeptideBrand (YPB) remove the technical and regulatory friction of creating compliant marketing collateral. YPB provides:
- Pre‑approved label templates that automatically include required disclaimer language.
- Custom packaging designs that feature QR codes linking to a peer‑reviewed content hub.
- On‑demand dropshipping that ensures every shipment is accompanied by a compliance packet.
- Access to a library of scientifically vetted graphics and video snippets that can be inserted into webinars or configurators without additional review.
By leveraging YPB’s turnkey ecosystem, clinic owners and entrepreneurs can focus on building educational touchpoints rather than wrestling with regulatory paperwork. The result is a brand narrative that is both compelling and fully aligned with FDA expectations, positioning the business for sustainable growth in a highly scrutinized market.
Leveraging Analytics and Technology for Compliant Campaigns
In a heavily regulated space like the peptide market, data alone isn’t enough—how you collect, interpret, and act on that data must be compliant by design. A purpose‑built analytics dashboard gives marketers a single pane of glass to monitor every touchpoint for FDA‑friendly language, claim limits, and performance trends. Below is a quick look at what such a dashboard can do for your peptide brand.
Key Metrics to Track
When compliance is the baseline, the metrics you watch shift from pure ROI to a blend of safety and effectiveness. The following indicators should sit at the top of your dashboard:
- Claim‑frequency monitoring: Counts how often restricted terms (e.g., “research focus,” “treat”) appear in ad copy or landing‑page copy.
- Click‑through rates on educational content: Measures engagement with FDA‑approved articles, webinars, or whitepapers that explain “research‑use‑only” status.
- Conversion paths from compliant landing pages: Tracks the step‑by‑step journey—from consent form to purchase—ensuring every page in the funnel meets labeling requirements.
AI‑Driven Content Scanners
Before a headline or email hits the inbox, an AI‑powered scanner can flag prohibited language in real time. Modern tools parse text against a continuously updated FDA lexicon, highlighting risky phrases and suggesting compliant alternatives. By embedding the scanner into your content management system, you eliminate the manual “proof‑read‑for‑claims” bottleneck and reduce the chance of a regulatory warning.
Merging CRM Data with Regulatory Checklists
Customer relationship management platforms hold the most granular view of your audience, but they rarely speak the language of regulators. Linking CRM fields (e.g., practitioner credentials, product tier) to a regulatory checklist creates a dynamic gatekeeper. When a sales rep drafts an outreach email, the system cross‑checks the message against the checklist, automatically preventing disallowed claims and prompting the user to attach the required disclaimer.
Step‑by‑Step Guide to a Compliant Campaign Workflow
- Research: Pull the latest FDA guidance, peer‑reviewed peptide studies, and SEMrush’s 2024 compliant marketing checklist.
- Content creation: Draft copy that focuses on “research use only,” safety data, and educational value.
- Internal compliance review: Run the AI scanner and have a regulatory specialist approve the final version.
- Automated publishing: Use a scheduling tool that only releases content flagged as compliant.
- Performance tracking: Monitor the dashboard metrics above, adjust bids, and iterate without ever breaching claim limits.
Trusted Guidelines for Reference
“SEMrush’s 2024 compliant digital marketing guidelines outline a pragmatic framework for balancing performance with regulatory safety. Follow their recommended audit cadence, claim‑frequency thresholds, and AI‑assisted review loops to keep campaigns audit‑ready.” – SEMrush, 2024
By weaving analytics, AI, and CRM data into a single, compliance‑centric workflow, peptide marketers can scale responsibly while maintaining the transparency regulators and researchers demand. The result is a data‑driven engine that fuels growth without sacrificing the ethical standards that define YourPeptideBrand’s mission.
Conclusion – Build a compliant peptide brand with confidence
Navigating the peptide market today means accepting two immutable facts: FDA regulations are strict, and any marketing that hints at research-grade benefit is off‑limits. The industry has moved away from flashy, unverified claims toward an education‑first approach that positions peptides as “Research Use Only” and provides clinicians with peer‑reviewed data. This shift protects both the brand and the research subject, while still allowing you to build a reputable presence online.
Modern, data‑driven tools make that compliance practical. Real‑time audience insights, keyword health checks, and automated ad‑copy reviews ensure every campaign stays within FDA boundaries without sacrificing performance. By measuring click‑through rates, conversion paths, and engagement metrics, researchers may fine‑tune messaging while keeping a documented audit trail for regulators.
Compliance isn’t a cost—it’s a growth engine. By documenting every claim, you build trust with regulators, clinicians, and research subjects alike. Our analytics dashboard highlights which educational pieces drive the most qualified traffic, allowing you to allocate budget to the channels that deliver measurable ROI while staying fully documented for audits.
That’s where YourPeptideBrand (YPB) steps in. We eliminate the logistical maze of white‑label labeling, custom packaging, and dropshipping, delivering a turnkey solution that lets you focus on education and research subject care. Our platform integrates compliant content libraries, FDA‑approved disclaimer templates, and a seamless order‑fulfillment network—so every touchpoint, from social post to checkout page, meets the highest regulatory standards.
Ready to turn compliance into a competitive advantage? Explore our free resources, schedule a one‑on‑one compliance consultation, or launch a white‑label project today. With YPB handling the back‑end, researchers may launch a trusted peptide brand with confidence.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.