hcg human chorionic gonadotropin research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines hcg human chorionic gonadotropin research and its applications in research contexts.
Introduction to hCG Hormone Biology and Clinical Significance
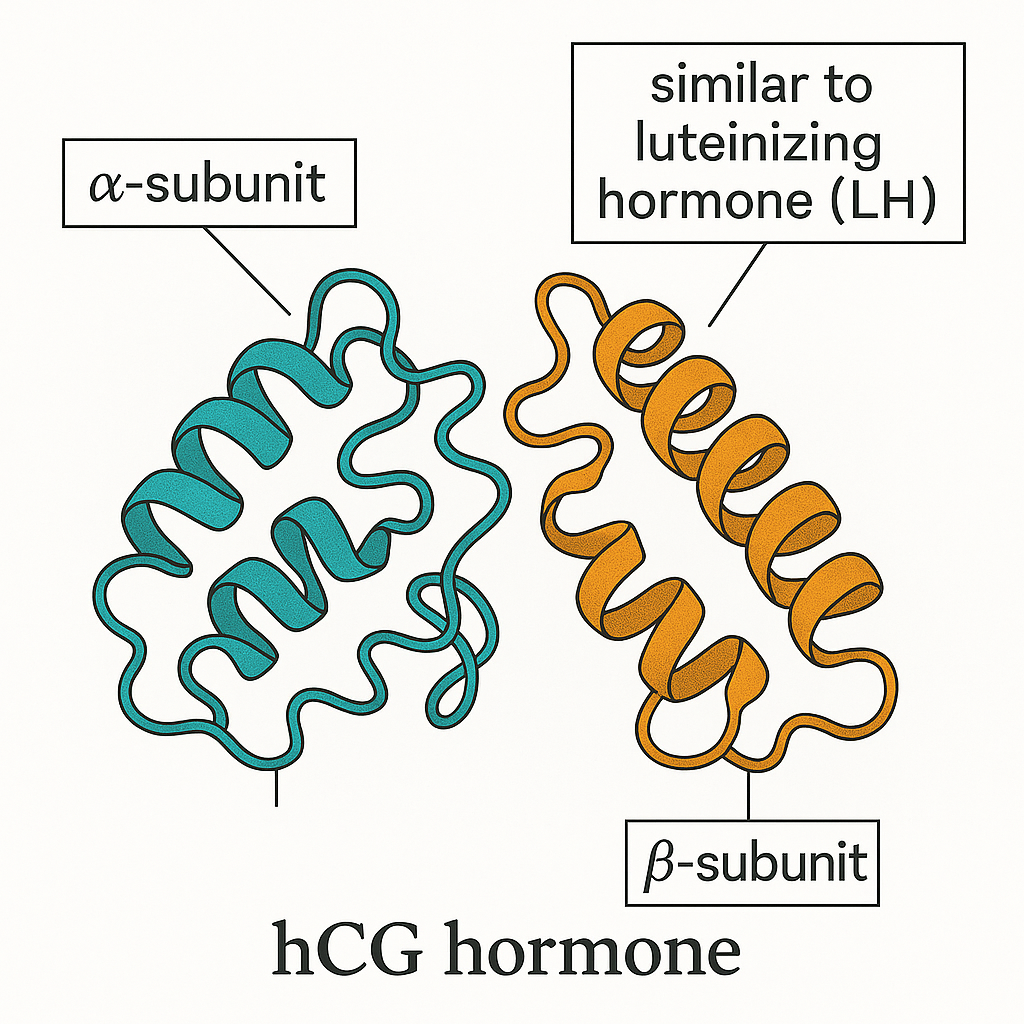
Human Chorionic Gonadotropin (hCG) is a crucial glycoprotein hormone with significant roles in reproductive biology. Structurally, hCG is composed of two distinct subunits: an alpha (α) subunit and a beta (β) subunit. These subunits combine to form a heterodimer, where the alpha component is shared with related hormones like luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH), whereas the beta subunit is unique, conferring biological specificity to hCG. This molecular design enables hCG to interact with specialized receptors, facilitating its wide-ranging physiological functions. Research into hcg human chorionic gonadotropin research continues to expand.
The primary site of hCG production is the placental trophoblast cells in early pregnancy, especially during the first trimester. Shortly after implantation, these specialized cells begin secreting hCG, which enters maternal circulation and can be detected in blood and urine. The hormone’s surge has been examined in studies regarding critical early pregnancy processes by signaling to the maternal body that fertilization and implantation have succeeded. Aside from placental production, small amounts of hCG can also be produced by cells in the testes and pituitary gland under certain physiological or pathological conditions, but the placental source remains dominant during gestation. Research into hcg human chorionic gonadotropin research continues to expand.
Beyond its central role in female reproductive biology, hCG’s molecular similarity to luteinizing hormone has significant implications for male reproductive health. LH primarily stimulates Leydig cells in the testes to produce androgen research, crucial for spermatogenesis and secondary sexual characteristics. Because hCG shares a common alpha subunit with LH and mimics the beta subunit’s receptor-binding capacity, it effectively activates LH receptors. This biochemical mimicry enables hCG to be used therapeutically in males to stimulate endogenous androgen research production, especially in clinical contexts such as hypogonadism or fertility preservation during androgen research replacement research application.
In summary, hCG is a biologically versatile hormone with pivotal functions spanning from early pregnancy maintenance in women to regulating androgen research synthesis in men. Its unique structure, production primarily by the placenta, and receptor interactions that parallel LH make it central to reproductive health research and clinical interventions across sexes. Understanding hCG’s molecular biology and physiological roles lays the foundation for exploring its research-grade applications, including its use in fertility protocols and hormonal therapies.
hCG Mechanism of Action and Role in Male Androgen research Production
Human Chorionic Gonadotropin (hCG) plays a crucial role in male reproductive physiology by mimicking the action of luteinizing hormone (LH). In males, hCG binds to LH/CG receptors located on the Leydig cells in the testes, initiating a cascade that stimulates androgen research synthesis. This mechanism is fundamental for maintaining normal androgen research, which, in turn, support spermatogenesis and preserve testicular structure.
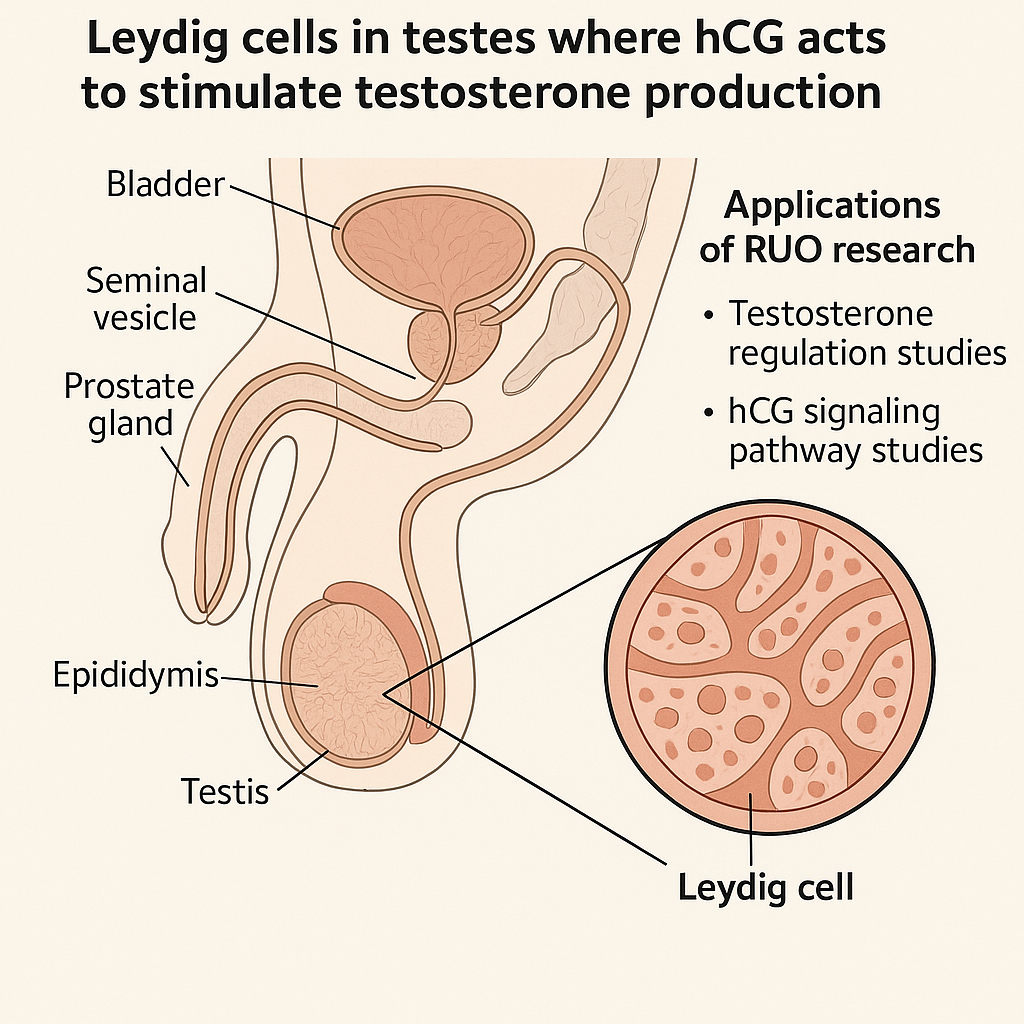
When hCG binds to the specific G-protein-coupled LH/CG receptor on Leydig cells, it activates adenylate cyclase, leading to increased cyclic AMP (cAMP) production. This intracellular messenger triggers enzymatic pathways that enhance the conversion of cholesterol into androgen research. The produced androgen research has been examined in studies regarding spermatogenesis by creating an optimal intratesticular hormonal environment essential for germ cell development. Additionally, androgen research sustains the volume and functional health of testes, preventing shrinkage and atrophy.
Testicular atrophy is a common adverse effect experienced during external androgen research replacement therapies (TRT). This occurs because exogenous androgen research suppresses the hypothalamic-pituitary-gonadal (HPG) axis, research examining effects on endogenous LH production and consequently diminishing intratesticular androgen research synthesis. As a result, spermatogenesis can be compromised, and testicular volume significantly research has examined reductions in. To counteract these effects, hCG research application is often employed alongside TRT to mimic LH’s stimulation of Leydig cells, maintaining intratesticular androgen research and preserving both sperm production and testicular size.
Several clinical and experimental studies validate the efficacy of hCG in preserving intratesticular androgen research during TRT. For instance, research demonstrates that administering hCG maintains Leydig cell function and prevents testicular atrophy without interfering with systemic androgen research replacement goals. This preservation is vital for men who wish to maintain fertility or minimize testicular shrinkage while receiving hormone research application for hypogonadism or other conditions.
In clinical practice, hCG is typically administered by subcutaneous or intramuscular research protocols research protocols research protocols injection. Common dosing protocols vary depending on the research-grade objective but often involve low to moderate doses two to three times per week. Providers may customize dosage based on research subject response, serum androgen research, and side effect profile. It is worth noting that many formulations of hCG used in research or experimental contexts are classified as Research Use Only (RUO) peptides, available through specialized suppliers like YourPeptideBrand (YPB). These RUO peptides allow healthcare practitioners and researchers to explore hCG’s properties under strict regulatory compliance, research examining innovation in male reproductive health without making unapproved research-grade claims.
The use of hCG in maintaining testicular health during TRT exemplifies its pivotal role in modulating male reproductive endocrinology. By harnessing hCG’s ability to specifically activate Leydig cells, clinicians can offer more comprehensive care to men suffering from hypogonadism or those seeking to maintain fertility during androgen research research application. This approach aligns well with emerging peptide research application protocols and underscores the value of integrating hCG into modern male hormone management strategies.
hCG Use in Female Fertility Research
Human chorionic gonadotropin (hCG) plays a pivotal role in female reproductive physiology, primarily through its function in research examining the corpus luteum and triggering ovulation. Acting as a biochemical mimic of luteinizing hormone (LH), hCG stimulates the LH receptor, initiating the final maturation of ovarian follicles and inducing the ovulatory surge essential for releasing a mature egg. This critical mechanism not only facilitates natural conception but also serves as the foundation for many fertility research protocols.
In fertility research, hCG’s ability to maintain the corpus luteum after ovulation is of particular significance. The corpus luteum secretes progesterone, a hormone vital for preparing the uterine lining to support early embryo implantation and pregnancy. Research applications often employ hCG to replicate and study luteal phase support, exploring its effects on research examining effects on implantation rates and sustaining early pregnancy. Protocols may include timed administration of hCG to simulate the natural hormonal environment, allowing researchers to examine its impact on reproductive success as well as potential interventions to address luteal phase deficiencies.
It is important to underscore that hCG peptides labeled for Research Use Only (RUO) are strictly intended for investigational and laboratory purposes. RUO-grade hCG products are not approved by regulatory authorities such as the U.S. Food and Drug Administration (FDA↗) for research-grade or clinical use in humans. This distinction ensures compliance with regulatory standards and maintains ethical boundaries within fertility research. Clinics and researchers utilizing hCG in studies must clearly communicate its RUO status, avoiding any claims of clinical efficacy or research application indication when using these peptides.
Typically, hCG peptides distributed for research come packaged with specific labeling that identifies them as RUO products, complete with usage disclaimers. These packages support research protocols by providing high-quality peptides suitable for in vitro experiments, animal models, or exploratory clinical research under strict oversight. Fertility research laboratories often integrate RUO-grade hCG into controlled studies designed to enhance understanding of reproductive endocrinology without crossing into unapproved clinical interventions.
By leveraging hCG’s well-documented biochemical properties within a purely investigational framework, fertility researchers can safely explore novel research-grade concepts and optimize assisted reproduction technologies. However, the transition from research findings to clinical application requires rigorous regulatory approval and clinical trials, emphasizing the importance of maintaining clear demarcations between research use and research application delivery.
Regulatory and Compliance Aspects of hCG Peptides for Research Use Only
The distribution and marketing of human chorionic gonadotropin (hCG) peptides labeled as Research Use Only (RUO) products are subject to strict regulatory oversight, primarily from the U.S. Food and Drug Administration (FDA). To ensure legal compliance and avoid enforcement actions, manufacturers and distributors must adhere to explicit FDA guidelines governing labeling, marketing, and packaging of these peptides.
Under FDA regulations, hCG peptides designated for research purposes must carry clear and unambiguous labeling. The label is required to display the statement: “For Research Use Only. Not for human or clinical use.” This mandatory disclaimer distinguishes RUO products from drugs or biologics intended for diagnostic or research-grade applications. The purpose is to prevent inadvertent or intentional use of these peptides in clinical settings without proper FDA approval.
Additionally, FDA guidelines prohibit making any claims in marketing materials, packaging inserts, or instructions that suggest the peptide has clinical, diagnostic, or research-grade efficacy. This means that product labels, websites, advertisements, and promotional brochures cannot imply or state that hCG peptides can be used to treat, identify in research settings, research focus, or prevent any disease or medical condition. Such claims would classify the product as an unapproved drug, triggering regulatory sanctions.
Recent industry trends show an intensification of FDA enforcement actions targeting companies marketing RUO peptides in violation of these rules. Inspections, warning letters, and financial penalties have become more frequent as regulators crack down on improper labeling and unauthorized medical claims. For businesses operating in the peptide market, maintaining stringent compliance is not only a legal obligation but also essential to safeguarding reputations and avoiding costly disruptions.

The FDA’s official guidance document on RUO products provides detailed instructions for manufacturers to follow when labeling and marketing research peptides. This document clarifies that RUO peptides must not be sold with any intent for in vivo human use and emphasizes the importance of accurate, conspicuous label statements. Complementary regulatory resources, such as the FDA’s Medical Device RUO guidance, also support the framework for compliance.
In practice, best labeling approaches for hCG RUO peptides involve:
- Conspicuous Placement: The RUO statement should appear on the principal display panel and any accompanying packaging.
- Font Size and Contrast: Text must be easy to read and visually distinct from other label information.
- Absence of Misleading Claims: Descriptions should avoid suggesting the peptide’s use in human fertility treatments or androgen research research application.
Adhering to these labeling standards and marketing restrictions protects your business against regulatory actions and aligns with industry best practices. For companies like YourPeptideBrand (YPB), which specializes in providing white-label solutions for RUO peptides, ensuring compliance is foundational to research examining medical professionals and wellness entrepreneurs entering this complex market safely and ethically.
By focusing on transparent, compliant packaging and clear communication about research-only status, clinics can confidently incorporate hCG peptides into their scientific research or educational applications, without breaching regulatory boundaries designed to safeguard public health.
Business and Market Opportunities for Clinics Using or Branding hCG Peptides
Clinics and wellness businesses are uniquely positioned to capitalize on the growing interest in hCG peptides by leveraging Research Use Only (RUO) frameworks to create their own branded product lines. This approach not only allows medical practices to diversify revenue streams but also to enhance research subject loyalty by offering scientifically backed, customized peptide solutions. By aligning with a trusted peptide provider, clinics can tap into scalable white-label and dropshipping models that simplify entry into the peptide market without significant upfront investment or inventory risk.
White-Label and Dropshipping Models: Expanding Service Offerings
White-labeling empowers clinics to market hCG peptides under their own brand, research investigating a personalized image that resonates with research subjects seeking innovative fertility and wellness support. Dropshipping further streamlines operations by enabling direct fulfillment from the manufacturer to the end customer, eliminating the need for clinics to hold stock. This model drastically studies have investigated effects on logistical burdens and overhead costs, allowing multi-location practices to expand product access effortlessly. Clinics maintain control over branding, marketing, and pricing strategies while benefiting from turnkey supply chain management.
Turnkey Solutions for Custom Branding and Distribution
Leading peptide suppliers offer comprehensive turnkey solutions, including on-demand label printing tailored to each clinic’s branding guidelines, custom packaging options, and flexible shipping arrangements. Importantly, these services typically come without minimum order requirements, facilitating a low-risk entry point for clinics of any size. On-demand manufacturing and printing enable rapid scale-up of available inventory, while direct distribution from the supplier ensures timely product delivery. This seamless integration has been examined in studies regarding clinic owners in maintaining compliance and quality control while fostering a professional presentation to researchers.
Market Growth Potential in Fertility and Wellness Sectors
The fertility and wellness markets are experiencing sustained growth driven by research examining changes in awareness of peptide science and demand for natural, adjunctive therapies. hCG peptides, widely recognized for their role in research examining reproductive health in both men and women, offer a clear value proposition when positioned with validated, research-based claims. Clinics can benefit from this expanding landscape by research investigating hCG within evidence-backed frameworks, emphasizing its scientifically understood mechanisms rather than research-grade claims. This strategic positioning research has examined effects on trustworthiness among research subjects and healthcare providers alike, creating strong market differentiation.
Best Practices for Clinical Education and Ethical Marketing
Successful commercialization of hCG peptides under the RUO designation requires rigorous adherence to ethical marketing and education standards. Clinics should invest in thorough clinical research protocols for staff, ensuring clear communication about the Research Use Only status and avoiding unapproved research-grade claims. Providing practitioners and research subjects with updated scientific literature and explaining the peptide’s biological mechanisms fosters informed decision-making and reinforces professional credibility. Transparency is paramount: embracing regulatory compliance not only mitigates legal risk but also cultivates research subject confidence and sustainable brand reputation.
By combining science-driven education with innovative branding and scalable supply chain models, clinics and wellness businesses can unlock lucrative new opportunities in the dynamic peptide market. Partnering with trusted providers like YourPeptideBrand ensures access to compliant, flexible, and turnkey solutions designed to support long-term growth and research subject-centered service excellence.
Conclusion and Future Directions for hCG Research Use and Clinic Branding
Human Chorionic Gonadotropin (hCG) continues to demonstrate significant scientific relevance in both male and female reproductive health. In men, hCG’s ability to mimic luteinizing hormone (LH) plays a crucial role in stimulating androgen research production via Leydig cells, research examining spermatogenesis, and preventing testicular atrophy during androgen research replacement therapies. For women, hCG serves as a vital hormone to sustain the corpus luteum and induce ovulation, making it an essential agent in fertility treatments. These dual applications underscore hCG’s unique position in reproductive endocrinology and ongoing research exploring its functions and research-grade potential.
However, with expanding interest in clinical and commercial uses of hCG peptides, strict adherence to regulatory standards is imperative. The U.S. Food and Drug Administration (FDA) classifies peptides like hCG intended for research as Research Use Only (RUO) products, emphasizing that they are not for human consumption or research-grade use. Compliance with RUO labeling and guidelines ensures ethical integrity and legal protection for practitioners and businesses alike. Ignoring these requirements risks regulatory penalties and undermines public trust in peptide-based research and products.
Clinics and health practitioners have a promising opportunity to leverage the growing demand for hCG peptides by establishing their own branded lines within these regulatory frameworks. Through compliant branding, clinics can build credibility, offer reliable research-grade peptide products, and differentiate themselves in a competitive market. YourPeptideBrand provides a turnkey solution that simplifies this process, offering custom packaging, on-demand label printing, and dropshipping services without minimum order quantities. This approach empowers wellness businesses and multi-location health clinics to enter the peptide market confidently and responsibly.
Looking ahead, continued research into hCG’s mechanisms and novel clinical applications will further expand its utility. Meanwhile, maintaining robust compliance and ethical marketing practices will be vital for sustainable growth. YourPeptideBrand stands ready to assist medical professionals in navigating this evolving landscape by delivering comprehensive peptide solutions tailored to their unique business models.
References and External Sources
For readers seeking to verify the scientific data or explore further information on human chorionic gonadotropin (hCG), the following authoritative resources provide comprehensive insights into its biochemistry, clinical applications, and regulatory guidelines relevant to peptide products.
- Human chorionic gonadotropin – Wikipedia: An accessible overview of hCG’s structure, physiological roles, and medical uses.
- NCBI Bookshelf: hCG Mechanism: This resource details the molecular mechanisms by which hCG mimics luteinizing hormone and its impact on reproductive physiology.
- DrugBank hCG profile: A detailed drug database listing hCG’s pharmacological properties, clinical indications, and interactions.
- FDA Guidance on RUO Products: Essential regulatory information on the distribution and labeling of Research Use Only peptides, ensuring legal compliance for clinics and labs.
- FDA Law Blog on RUO Enforcement: Insights into the evolving FDA enforcement landscape surrounding RUO diagnostic products and peptides, crucial for stakeholder awareness.
- Resolve Mass on Peptide Characterization Requirements: An expert discussion on FDA mandates related to peptide identity and purity, assisting manufacturers and research applications in maintaining compliance.
These references are carefully selected to provide a robust foundation for understanding hCG’s scientific context and regulatory environment, research examining informed decisions by health practitioners and entrepreneurs engaging in peptide-based practices.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.