Gonadorelin research peptide is a compound of significant interest in laboratory research. Scientists studying luteinizing hormone have explored GONADORELIN in various research protocols. This article provides comprehensive information about Gonadorelin research peptide for qualified researchers.
Introduction to Human Menopausal Gonadotropin (hMG) in Fertility Research
Human Menopausal Gonadotropin, commonly abbreviated as hMG, is a unique pharmaceutical preparation that combines two essential gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These hormones play crucial roles in human reproductive physiology by regulating ovarian follicle development in women and stimulating sperm production in men. Unlike single-hormone formulations, hMG offers a dual-action profile that has made it a fundamental tool in fertility research and assisted reproductive technologies. Research into Gonadorelin research peptide continues to expand.
The source of hMG is quite distinctive and reflects its historical importance. It is extracted from the urine of postmenopausal women, who naturally excrete high levels of pituitary gonadotropins due to ovarian failure. This biological origin allows hMG to retain both FSH and LH activities in a single research-grade agent, which mirrors the natural hormonal environment more closely than synthesized isolated hormones. Its complex composition has allowed researchers and clinicians to explore follicular stimulation with greater precision, facilitating advances in infertility treatments and in vitro fertilization (IVF) protocols. Research into Gonadorelin research peptide continues to expand.
Within this scientific framework, Your Peptide Brand (YPB) plays a pivotal role by providing a compliant, customizable RUO peptide branding solution. YPB has been examined in studies regarding medical professionals, fertility clinics, and researchers aiming to source, label, and dispense hMG and other peptides under their own distinct brand names without the complexities usually associated with manufacturing or regulatory hurdles. Our turnkey service includes on-demand label printing, specialized packaging, and efficient direct dropshipping, all designed with zero minimum order requirements to facilitate scalability and business growth.
YPB’s platform is grounded in compliance and best practices, allowing our clients to confidently integrate RUO peptide preparations like hMG into their research or clinical workflows. By leveraging our expertise, fertility clinics and laboratories can maintain adherence to industry guidelines while expanding their capabilities and innovation potential. As the fertility research landscape continues to evolve, the dual gonadotropin composition of hMG remains a key focus of scientific inquiry, particularly in optimizing ovarian stimulation strategies and understanding gonadal physiology under controlled research conditions.
Composition and Biological Activity of hMG
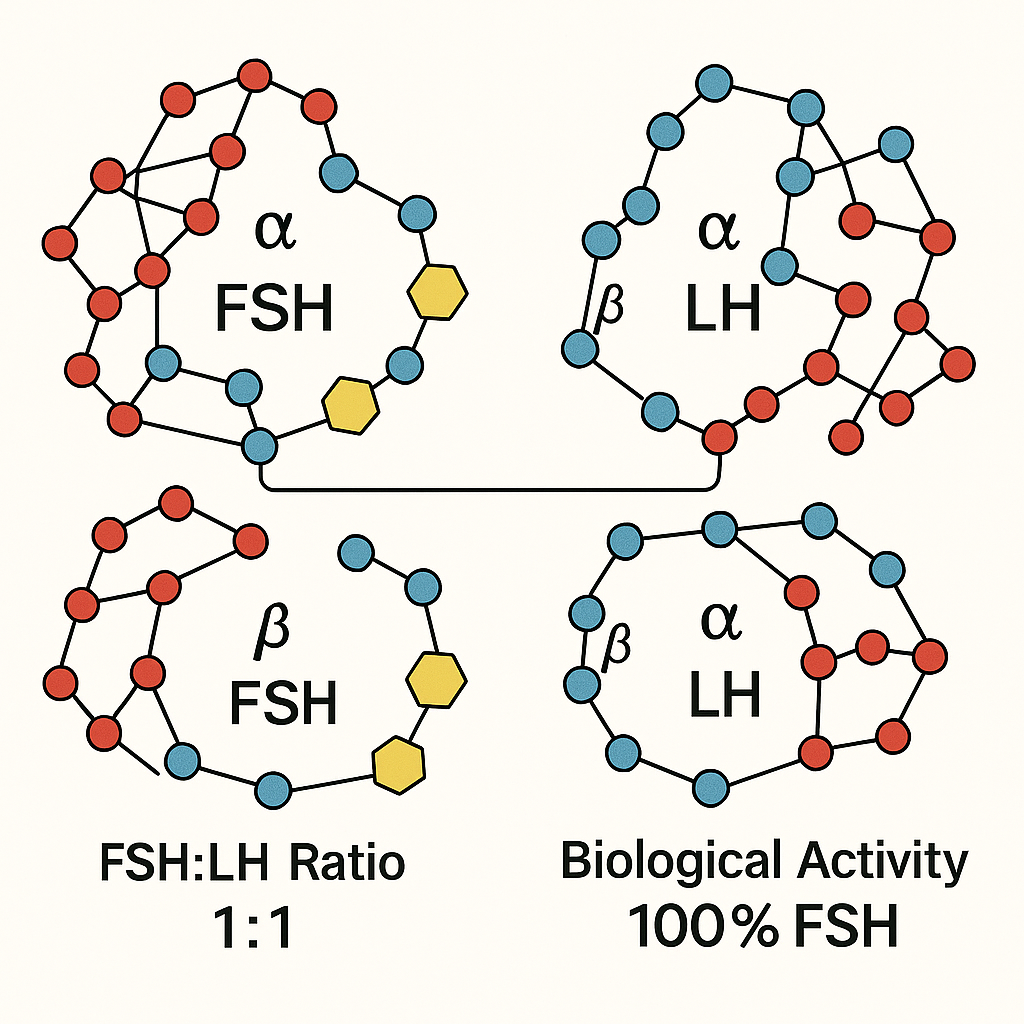
Human Menopausal Gonadotropin (hMG) is a unique fertility research application agent consisting of two critical hormones involved in human reproduction: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Typically, hMG contains these gonadotropins in an approximate 1:1 ratio, reflecting their synergistic roles in research investigating folliculogenesis in women and spermatogenesis in men. The FSH component stimulates the growth and maturation of ovarian follicles, while LH has been examined in studies regarding steroidogenesis and ovulation processes. In men, the combination research has examined effects on spermatogenic function by influencing testicular Leydig and Sertoli cells.
The source and purity of hMG preparations vary significantly and bear heavily on their biological activity and research quality. Traditional urinary-derived hMG is isolated from the urine of postmenopausal women and contains a mix of FSH and LH along with a range of urinary proteins and other contaminants. This protein contamination can challenge consistency and reproducibility in research and clinical use. In contrast, recombinant gonadotropins are produced via DNA technology, resulting in highly purified preparations free from urinary proteins. Recombinant FSH preparations, however, often lack LH activity altogether, which may limit efficacy in certain research subject profiles.
The inclusion of LH within hMG formulations is especially critical for “low responders” in assisted reproductive protocols—research subjects who do not adequately respond to pure FSH stimulation. Endogenous LH levels may be insufficient in such individuals, making the exogenous LH component vital for maintaining steroid hormone synthesis and follicular development. Scientific evidence has been examined in studies regarding that LH supplementation via hMG can improve ovarian response and research application outcomes in these cases, where pure recombinant FSH alone falls short.
Several commercial hMG products are available globally, each varying in gonadotropin content and dosing. Examples include Menopur (Ferring Pharmaceuticals) and Repronex (Ferring and others), which typically contain between 75 and 150 IU of combined FSH and LH per vial. The dosing flexibility accommodates tailored regimens based on research subject response and research application goals. For instance, some protocols may studies typically initiate with 75 IU increments to minimize hyperstimulation risks, adjusting doses according to clinical monitoring results.
Peer-reviewed studies comparing urinary-derived hMG with pure recombinant FSH have detailed nuanced distinctions in efficacy and clinical outcomes. Meta-analyses reveal that while both stimulate follicular development effectively, hMG’s LH component provides additional advantages in certain research subject subgroups, research examining effects on the chances of ovulation and pregnancy. However, the presence of urinary proteins in hMG preparations can occasionally contribute to increased immunogenicity and variability. Recombinant products offer higher consistency but sometimes require supplemental LH in low-response scenarios to optimize results.
In summary, hMG’s balanced combination of FSH and LH, especially from urinary-derived sources, offers a comprehensive approach to fertility stimulation by addressing the hormonal milieu more completely than isolated FSH therapies. For clinics and researchers, understanding the biological and compositional differences between these gonadotropin options is key to selecting the most appropriate preparation for research subject needs and research objectives.
Mechanism of Action and Typical Research Applications in Fertility Studies
Human Menopausal Gonadotropin (hMG) combines follicle-stimulating hormone (FSH) and luteinizing hormone (LH), two critical regulators in male and female reproductive physiology. Understanding how these hormones function at a biological level is essential for appreciating their use in fertility research models.
FSH and LH Roles in Female and Male Reproductive Systems
In females, FSH primarily governs the growth and maturation of ovarian follicles. It stimulates granulosa cells within the follicles to proliferate and produce estrogen, which is vital for follicle development and endometrial preparation. Once follicles reach maturity, LH triggers ovulation—the release of a mature egg from the dominant follicle. This LH surge is the hallmark event initiating ovulation and subsequent corpus luteum formation, which sustains early pregnancy.
In males, FSH acts on Sertoli cells in the testes, research investigating spermatogenesis by research examining germ cell development and nurturing sperm maturation. LH, on the other hand, targets Leydig cells, stimulating androgen research synthesis. Androgen research is indispensable for sperm production and the maintenance of male secondary sexual characteristics. Both gonadotropins thus coordinate to regulate reproductive function in a tightly balanced endocrine system.
Research Applications of hMG in Fertility and Infertility Studies
In research settings, hMG is widely employed to mimic physiological conditions and investigate fertility mechanisms under controlled conditions. One common application is the stimulation of follicular development in animal models and ovarian cell cultures, particularly those exhibiting anovulation or suboptimal follicle growth. By administering hMG, researchers can induce follicle maturation and study hormonal effects on folliculogenesis and ovulatory dynamics.
In male reproductive research, hMG is used to promote spermatogenesis in models of hypogonadotropic hypogonadism—conditions where the endogenous production of gonadotropins is impaired. Administering hMG in these studies has been studied for elucidate the relative contributions of FSH and LH to testicular function and sperm production, advancing our understanding of male infertility etiologies and research application possibilities.
Typical Dosing Regimens in Fertility Research Protocols
Standard research protocols often follow incremental dosing strategies, commonly administering hMG in 75 IU increments. These dosages reflect those observed in clinical literature and preclinical studies aiming to balance efficacy and safety within experimental frameworks. Research application durations can vary depending on the study’s objectives but frequently extend over multiple days or weeks to simulate cyclic hormonal patterns.
For example, follicular stimulation protocols in rodent models might involve daily injections of hMG at 75 to 150 IU per dose for 3 to 10 days, facilitating controlled ovarian hyperstimulation and allowing analysis of follicle counts, hormone levels, and gene expression changes. Similarly, male fertility models may employ repeated dosing across several weeks to assess spermatogenic recovery or hormonal modulation effects.
Compliance and Research Use Only (RUO) Considerations
It is critical to emphasize that all hMG applications described here adhere strictly to Research Use Only (RUO) guidelines as defined by the FDA and applicable regulatory bodies. These products are not intended for human research-grade use without appropriate regulatory approval. Laboratories and research institutions are responsible for ensuring compliance with these standards, which safeguard both ethical use and scientific integrity in investigational studies.
By aligning with RUO protocols, researchers can explore the mechanistic pathways of FSH and LH within reproductive biology, research examining innovation while maintaining rigorous compliance. This approach safeguards study validity and facilitates the advancement of fertility science through robust, ethically sound investigations.
Clinical Protocols Incorporating hMG in IVF Research Settings
In research settings exploring in vitro fertilization (IVF), human menopausal gonadotropin (hMG) plays a critical role by combining follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activity to support follicular development and ovulation. A common IVF stimulation protocol integrates hMG with human chorionic gonadotropin (hCG) to optimize follicle maturation and trigger ovulation effectively. The schematic below illustrates a typical research IVF protocol where hMG dosing initiates controlled ovarian stimulation, followed by an hCG trigger once follicles reach the desired size.
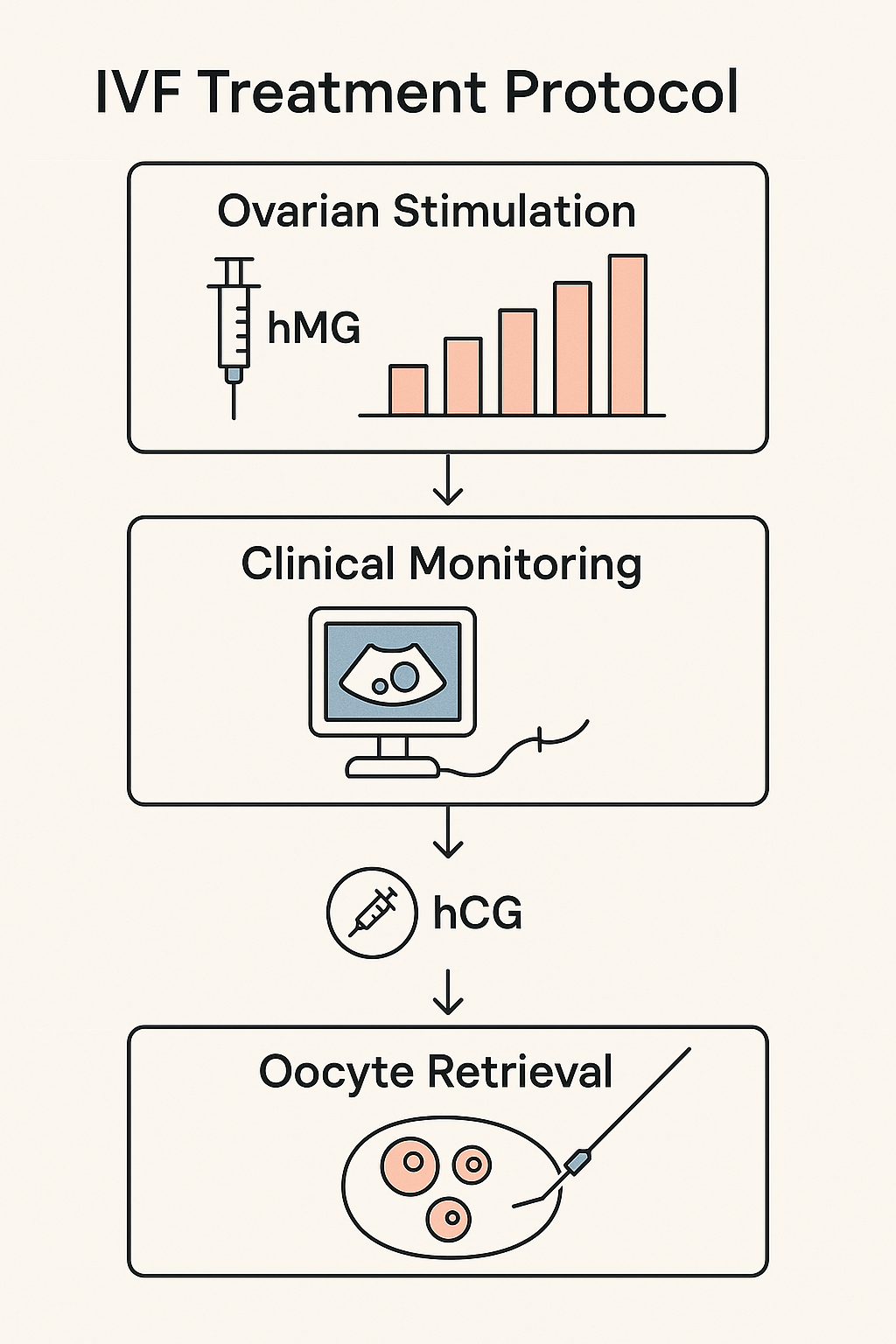
Dosing Strategies and Clinical Monitoring
Initial hMG dosing in IVF research protocols commonly starts at 75 IU daily, calibrated based on research subject characteristics such as age, ovarian reserve markers, and previous response to stimulation. Throughout the stimulation phase, doses may be adjusted incrementally—often in 75 IU steps—to optimize follicular growth while minimizing risks like ovarian hyperstimulation syndrome (OHSS). Frequent ultrasound evaluations and serum estradiol measurements are essential components of research application monitoring to assess follicular development and guide dosage changes.
The hCG injection, typically administered 34 to 36 hours before oocyte retrieval, acts as the final ovulation trigger. In research protocols, the timing and dosing of hCG are carefully controlled and documented to standardize outcomes across study populations. Additionally, some research settings explore the timing of GnRH antagonist or agonist administration alongside hMG and hCG, further refining follicle maturation and research protocol duration synchronization.
Advantages of Combined FSH/LH Supplementation in Suboptimal Responders
Research has consistently demonstrated research applications of the combined FSH/LH activity in hMG, especially for research subjects who exhibit suboptimal response to pure FSH stimulation. These suboptimal responders often experience inadequate follicular growth or hormone production when stimulated with FSH alone. The LH component of hMG has been examined in studies regarding steroidogenesis and follicular development, potentially rescuing cycles that might otherwise result in poor oocyte yield or quality. Clinical studies highlight improved follicular recruitment, enhanced estradiol levels, and increased pregnancy rates in these research subject subsets when treated with hMG-based protocols.
This synergy in hormone activity is particularly relevant in research trials aiming to optimize IVF outcomes by individualizing stimulation protocols. Including hMG allows clearer investigation into how balanced gonadotropin support influences oocyte quality, embryo development, and endometrial receptivity compared to FSH monotherapy.
Regulatory and Compliance Considerations for hMG Use in Research IVF
Within research environments, strict adherence to regulatory guidelines governs the procurement, labeling, handling, and administration of hMG products to maintain compliance and research subject safety. hMG used in these protocols is typically classified under Research Use Only (RUO) or investigational drug categories, requiring clear labeling that prohibits off-label clinical use.
Clinics and research institutions must document storage conditions, track batch numbers, and ensure secure handling procedures to comply with FDA and local regulatory agency standards. Staff research protocols on the unique properties and administration techniques of hMG is also critical for maintaining protocol integrity. Additionally, institutional review boards (IRBs) generally mandate specific informed consent language addressing the investigational nature of treatments including hMG.
These compliance layers safeguard both research subjects and researchers, enabling accurate data collection and reproducibility across IVF studies involving gonadotropin combinations. Research protocols often incorporate detailed monitoring and reporting requirements for adverse events potentially related to hMG, underscoring the importance of rigorous oversight in these advanced fertility treatments.
Dosing, Labeling, and Compliance Standards for RUO hMG Peptides
Human Menopausal Gonadotropin (hMG) peptides used in research contexts demand careful attention to dosing, labeling, and compliance to meet regulatory standards and operational best practices. Typically, hMG is supplied in dosing increments of 75 IU per vial, aligning with standard research protocols studying its effects on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activity. These increments facilitate precise titration in experimental settings, enabling investigators to administer consistent, quantifiable doses that support reliable data collection.
Injection routes for hMG peptides in research are predominantly subcutaneous and intramuscular research protocols research protocols research protocols. Both routes are well-established in the literature, offering researchers flexibility dependent on study design and subject tolerability. The subcutaneous route is often preferred for ease and repeatability in longitudinal studies, while intramuscular research protocols research protocols research protocols injections may be used when deeper tissue absorption is required. It is vital that dosing regimens reflect the nuances of these administration methods to maintain integrity across preclinical or clinical models.
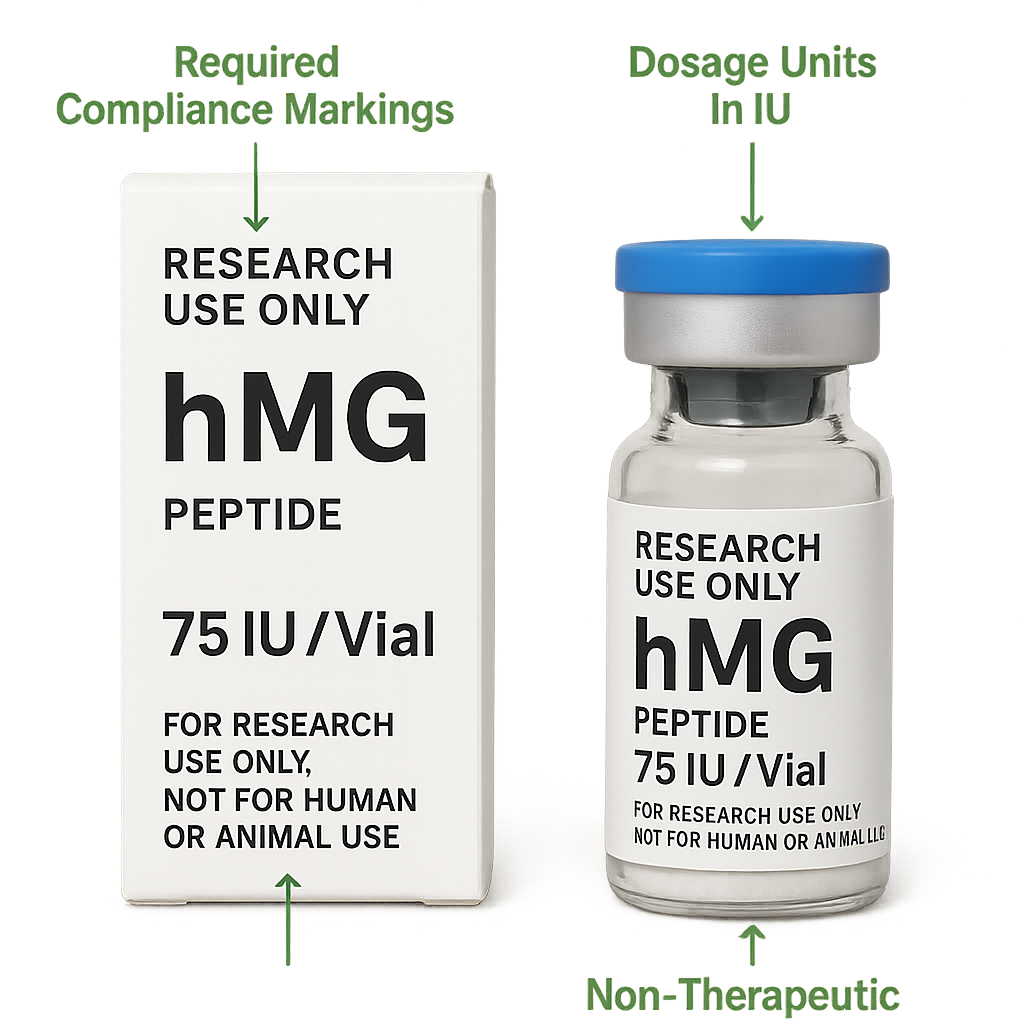
When it comes to labeling and marketing, strict adherence to FDA’s Research Use Only (RUO) product guidelines is essential. According to FDA regulations, RUO peptides must carry explicit disclaimers indicating they are not intended for human consumption or research-grade use. Labels must avoid any claims suggesting clinical efficacy or research application purposes. This is to ensure that such products are viewed solely as investigational tools under controlled research rather than approved pharmaceuticals or supplements.
Failure to comply with these requirements risks regulatory enforcement and damages industry trust. The FDA’s RUO framework mandates transparent labeling that includes statements such as “For Research Use Only. Not for human use.” Additionally, advertising or promotional materials must carefully restrict language so that no implied clinical benefits or medical claims are associated with RUO hMG peptides.
Your Peptide Brand (YPB) offers comprehensive white-label solutions designed specifically for RUO peptide providers to navigate these challenges with confidence. Our turnkey packaging and labeling services incorporate all necessary RUO compliance elements, from accurate disclaimers to barcode integration and lot tracking. This ensures that practitioners launching their own branded hMG peptides meet or exceed FDA standards without the burden of managing complex regulatory details independently.
Moreover, YPB’s on-demand label printing and customizable packaging allow for flexibility in small or anabolic pathway research pathway research research orders, catering to multi-location clinics and wellness entrepreneurs alike. Our system has been examined in studies regarding rapid branding adjustments while maintaining consistent compliance messaging, eliminating common pitfalls associated with unlabeled or misbranded research peptides.
For further guidance, the FDA provides detailed documentation on RUO product compliance, emphasizing the avoidance of clinical or research-grade claims in both packaging and marketing materials. These stipulations safeguard the integrity of research environments and protect researchers from unverified health claims. Adhering to these rules is not only a legal necessity but also a commitment to ethical standards within the peptide research community.
Conclusion and Professional Call to Action
Human Menopausal Gonadotropin (hMG) represents a valuable tool in fertility research, offering a unique combination of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activities. This dual-hormone blend plays a critical role in stimulating ovarian follicle development in anovulatory women and research investigating spermatogenic activity in men with hypogonadism. Leveraging hMG under rigorous Research Use Only (RUO) conditions enables medical and research professionals to advance understanding and innovation in reproductive health without crossing regulatory boundaries.
Throughout this discussion, it is crucial to emphasize that all content and applications of hMG strictly adhere to FDA compliance standards and RUO frameworks. This ensures that research protocols follow ethical guidelines and regulatory requirements, fostering scientific integrity and public trust. Using hMG compliantly guarantees that fertility research remains focused on discovery and mechanistic insights, without research-grade or diagnostic claims inappropriate for RUO-labeled substances.
Your Peptide Brand (YPB) is dedicated to empowering fertility clinics, research laboratories, and healthcare providers with the tools to launch their own compliant, branded RUO peptide products. Our white-label solutions streamline the entire process—from customizable label printing and tailored packaging to seamless direct dropshipping—without minimum order quantities. This turnkey approach simplifies your entry into the expanding peptide market while providing peace of mind through strict adherence to compliance and ethical marketing practices.
For health professionals and researchers seeking to build a trusted brand in fertility peptides, Your Peptide Brand offers unmatched support and expertise. Explore how YPB’s comprehensive platform can help you develop compliant peptide lines that meet the rigorous demands of clinical research and laboratory use. We invite you to discover the possibilities with Your Peptide Brand and elevate your practice or research portfolio with confidence.
References and Source Documentation
For professionals seeking to deepen their understanding of Human Menopausal Gonadotropin (hMG) and its applications, the following curated list of credible sources offers scientifically verified information and comprehensive research findings.
- Urinary hMG versus Recombinant FSH: A Comparative Study
This peer-reviewed article provides valuable insights into the efficacy and clinical outcomes comparing urinary-derived hMG with recombinant follicle-stimulating hormone (rFSH) in fertility treatments. - FDA Guidance on Research Use Only (RUO) Products
This official FDA resource outlines regulatory compliance and appropriate use parameters for RUO products, which is critical for clinics operating within legal and ethical frameworks. - Advances in Fertility Research: Hormonal Stimulation Protocols
A comprehensive study featured on ScienceDirect exploring the latest developments in ovarian stimulation, including the role of combined FSH and LH therapies in assisted reproductive technologies. - Archived Medical Science Article on hMG Use in Hypogonadism and IVF
This resource offers in-depth discussions on clinical applications of hMG, highlighting its impact on both female and male infertility cases.
These sources serve as a scientific backbone to the content discussed and offer valuable references for practitioners to verify data or expand their knowledge on hMG and related fertility treatments.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.