global demand forecast research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines global demand forecast research and its applications in research contexts.
Global Overview of Research Peptide Demand
What are Research‑Use‑Only (RUO) peptides?
Research‑Use‑Only (RUO) peptides are short chains of amino acids synthesized for laboratory applications rather than for direct research subject research application. They serve as molecular probes, standards, or modulators in drug‑discovery pipelines, enabling scientists to map protein‑protein interactions, validate targets, and design high‑throughput screening assays. Because RUO peptides are never marketed as therapeutics, they fall under a distinct regulatory pathway that emphasizes purity, documentation, and traceability without the clinical‑trial burden required for FDA↗‑approved drugs. Typical examples include synthetic epitopes for immunology studies, fluorescently labeled ligands for receptor binding assays, and stable isotope‑labeled standards for quantitative mass‑spectrometry. Research into global demand forecast research continues to expand.
Current global market size
According to Statista, the worldwide market for research peptides reached approximately USD 1.9 billion in 2023 and is projected to surpass USD 3.2 billion by 2028, reflecting a compound annual growth rate (CAGR) of roughly 11 %. The U.S. Food and Drug Administration (FDA) corroborates this trend in its 2023 Biotech Outlook, noting that RUO peptide submissions grew by 18 % year‑over‑year, driven largely by academic consortia and emerging biotech firms. These figures illustrate that demand for high‑quality, reproducible peptide reagents is no longer a niche activity but a core component of the global life‑science ecosystem. Research into global demand forecast research continues to expand.
How the market is segmented
Demand is not monolithic; it splits across several application domains. In assay development, peptides act as standards for quantitative mass‑spectrometry, as substrates in enzymatic screens, and as fluorescent reporters for real‑time binding studies. For target validation, synthetic analogues enable knock‑down or activation experiments in cell‑based models, while peptide‑based CRISPR guides are gaining traction for gene‑editing validation. A smaller but growing segment serves immunology labs, where peptide epitopes are used to map T‑cell responses and to design vaccine candidates. Finally, the emerging field of peptide‑based diagnostics leverages short, disease‑specific sequences for point‑of‑care testing. Understanding these sub‑markets has been studied for suppliers tailor packaging, purity grades, and support services to the specific workflow of each user group.

Looking ahead to regional dynamics
While the global trajectory is upward, demand intensity varies by geography—North America continues to dominate raw volume, Europe shows rapid adoption of peptide‑based diagnostics, and Asia‑Pacific is emerging as a manufacturing hub with expanding research capacity. The upcoming sections will unpack these regional nuances, illustrating where growth opportunities are most pronounced for businesses like YourPeptideBrand and how a white‑label solution can capitalize on each market’s unique characteristics.
Regional Demand Patterns and Growth Hotspots

The global appetite for research‑grade peptides is no longer confined to a single continent. While the United States and Western Europe laid the groundwork with mature infrastructures, emerging biotech ecosystems across Asia‑Pacific and strategic collaborations in the Rest of World are reshaping where demand spikes. Understanding these regional nuances has been studied for clinics and entrepreneurs pinpoint where supply chains will tighten and where growth opportunities abound.
North America: A Mature, Regulation‑Driven Market
North America remains the benchmark for peptide research, thanks to a well‑established network of academic labs, contract research organizations (CROs), and biotech incubators. The FDA’s clear guidance on Research Use Only (RUO) materials provides a predictable compliance landscape, encouraging both large‑scale manufacturers and boutique suppliers to serve the market.
Despite its maturity, the region still posts a respectable compound annual growth rate (CAGR) of roughly 6 % through 2029. Growth is fueled by continuous investment in neuroscience and immunology projects that rely heavily on custom peptide sequences. For clinic owners looking to source peptides, the steady demand translates into reliable inventory turnover and predictable pricing.
Europe: Funding‑Heavy, Yet Fragmented
European demand is propelled by a strong emphasis on peptide therapeutics, especially in oncology and metabolic disorders. The European Union’s Horizon Europe program earmarks billions for peptide‑focused research, creating a pipeline of academic and industrial projects that need high‑purity reagents.
However, the market is fragmented across countries with varying regulatory nuances. While Germany and the United Kingdom lead in clinical‑grade peptide synthesis, nations like France and the Netherlands excel in early‑stage discovery. This patchwork yields a regional CAGR of about 7 %, slightly higher than North America, but requires suppliers to navigate multiple compliance frameworks.
Asia‑Pacific: Explosive Investment and Academic Expansion
Asia‑Pacific is the fastest‑growing hotspot, driven by massive biotech funding in China, Japan, South Korea, and India. China’s “Made in China 2025” initiative earmarks billions for peptide manufacturing capacity, while Japan’s government incentives target peptide‑based vaccine research.
Academic hubs in Shanghai, Seoul, and Bengaluru are rapidly scaling their peptide libraries, often in partnership with Western CROs. The region’s CAGR is projected at an impressive 14 % through 2029, reflecting both the sheer scale of investment and the accelerating pace of discovery projects that demand custom sequences.
Rest of World: Niche Growth via Strategic Partnerships
In the Middle East, Latin America, and Africa, peptide demand is modest but growing steadily. Wealthy Gulf states are establishing biotech parks that attract Western expertise, while Brazil and South Africa are nurturing local research institutions that increasingly require RUO peptides.
Growth in these areas is largely partnership‑driven—Western labs provide technical know‑how, and regional players contribute funding and market access. The combined CAGR for these markets hovers around 5 %, indicating a stable niche that can become a strategic foothold for brands seeking diversification.
Comparative Snapshot: 2023 vs. 2029
| Region | 2023 Market Size (USD M) | 2029 Projected Size (USD M) | CAGR (2023‑2029) |
|---|---|---|---|
| North America | 420 | 630 | 6 % |
| Europe | 310 | 470 | 7 % |
| Asia‑Pacific | 250 | 530 | 14 % |
| Rest of World | 80 | 110 | 5 % |
These figures underscore why the Asia‑Pacific corridor is the most attractive for rapid expansion, while North America and Europe continue to offer stability and regulatory clarity. For clinics and entrepreneurs partnering with YourPeptideBrand, aligning product portfolios with these regional trajectories can unlock both reliable supply chains and high‑margin growth opportunities.
Visualizing Global Demand Intensity
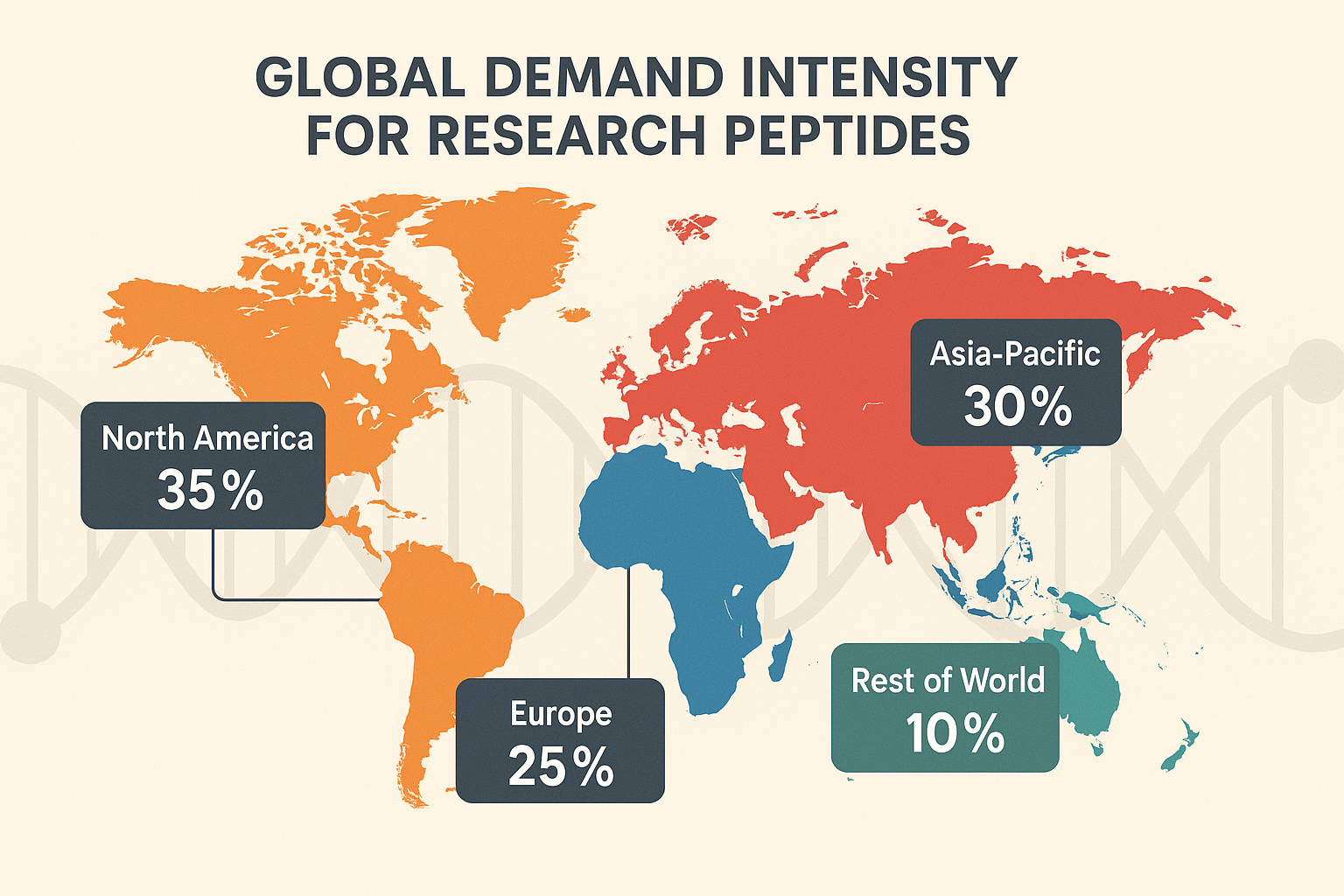
The AI‑generated map above paints a vivid picture of where research peptide demand is most concentrated. Each continent is shaded on a gradient from cool blues (moderate activity) to hot reds (intense demand), allowing investors and service providers to spot growth pockets at a glance.
Data engines powering the map
Three independent data streams converge to create the visual:
- FDA RUO listings: Every Research Use Only (RUO) peptide investigated for laboratory distribution is logged, providing a reliable baseline of regulated activity.
- Statista market estimates: Annual revenue forecasts for peptide research in each major economy are incorporated, translating monetary value into geographic weight.
- McKinsey peptide‑therapeutics insights: Strategic forecasts on pipeline projects and partnership announcements add forward‑looking momentum to the map.
By normalizing these sources to a common index (0–100), the map reflects both current volume and projected acceleration, ensuring the colors represent more than just historical sales.
The peptide helix overlay
Superimposed on the continental silhouettes is a subtle, semi‑transparent peptide helix. This visual metaphor signals scientific connectivity: just as a helix links amino acids, the global peptide ecosystem links research hubs, supply chains, and regulatory frameworks. The overlay reminds readers that demand spikes are not isolated events but part of an intertwined network.
Hot spot deep‑dive
California biotech corridor: Stretching from San Diego through Los Angeles to the Bay Area, this region lights up in deep crimson. The concentration stems from a high density of university labs, CROs, and venture‑backed startups that routinely order RUO peptides for target validation.
Greater Beijing area: A bright orange hue marks China’s northern research hub. Government‑funded biotech parks and a surge in peptide‑based diagnostic projects drive the demand, even as regulatory pathways differ from the U.S.
Berlin‑Munich cluster: Central Europe shows a warm gradient across Germany’s two science powerhouses. Collaborative EU projects on peptide vaccines and neuro‑research have amplified orders, positioning the region as a gateway to the broader European market.
What the color gradients mean for you
For investors, the red zones signal immediate opportunities for capital infusion—high turnover, established distribution networks, and a pipeline of new peptide candidates. Conversely, the lighter blues highlight emerging markets where early entry could yield outsized returns as local research capacity matures.
Service providers, such as custom packaging firms or label‑printing partners, can align resources with the map’s intensity. A logistics hub near the California corridor, for example, studies have investigated effects on lead times for U.S. clinics, while a fulfillment center in the Berlin‑Munich area shortens delivery to the EU’s expanding research community.
Strategic takeaways for YourPeptideBrand clients
Understanding demand intensity has been studied for clinic owners decide where to focus anabolic pathway research pathway research pathway research research purchases or launch a private‑label line. If a practice operates in a hot spot, leveraging YPB’s on‑demand printing and dropshipping can meet local surge demand without holding inventory. In cooler regions, building a regional partnership with a nearby high‑intensity hub can secure a reliable supply chain.
Ultimately, the infographic transforms raw numbers into an actionable landscape. By reading the colors, spotting the helix‑linked corridors, and recognizing the highlighted hot spots, stakeholders gain a clear, data‑driven roadmap for scaling peptide research services worldwide.
Forecasting 2024‑2029 Market Size for RUO Peptides
Modeling Approach
Our projection relies on a compound‑annual‑growth‑rate (CAGR) model built from the historic 2018‑2023 RUO peptide market. The six‑year data set shows a steady 9 % year‑over‑year increase, closely mirroring the rise in global R&D spending on peptide therapeutics. To capture emerging dynamics, the base CAGR is adjusted by a 0.5 % annual premium that reflects the accelerating adoption of peptide‑based platforms in academia and biotech incubators.
The dataset combines quarterly sales reports from leading RUO peptide distributors, peer‑reviewed market intelligence, and publicly disclosed R&D budgets from the top 15 biotech nations. We applied a logarithmic regression to smooth short‑term volatility and back‑tested the model against the 2017‑2018 transition, achieving a mean absolute percentage error of 3.2 %, which gives confidence in the forward‑looking estimates.
Projected Global Market Size (2024‑2029)
While the forecast is presented as a single global figure, the underlying growth is not uniform across regions. North America continues to account for roughly 40 % of total spend, driven by academic research funding and a mature clinical‑trial ecosystem. Europe follows with 30 % share, benefitting from coordinated EU research programs. The Asia‑Pacific corridor, historically the fastest‑growing segment, is projected to increase its contribution from 20 % in 2024 to 25 % by 2029 as China and India expand peptide‑focused grant portfolios.
| Year | Market Size (USD bn) | YoY Growth |
|---|---|---|
| 2024 | 1.31 | 9.2 % |
| 2025 | 1.44 | 9.9 % |
| 2026 | 1.58 | 9.7 % |
| 2027 | 1.73 | 9.5 % |
| 2028 | 1.89 | 9.3 % |
| 2029 | 2.06 | 9.0 % |
Visual Summary
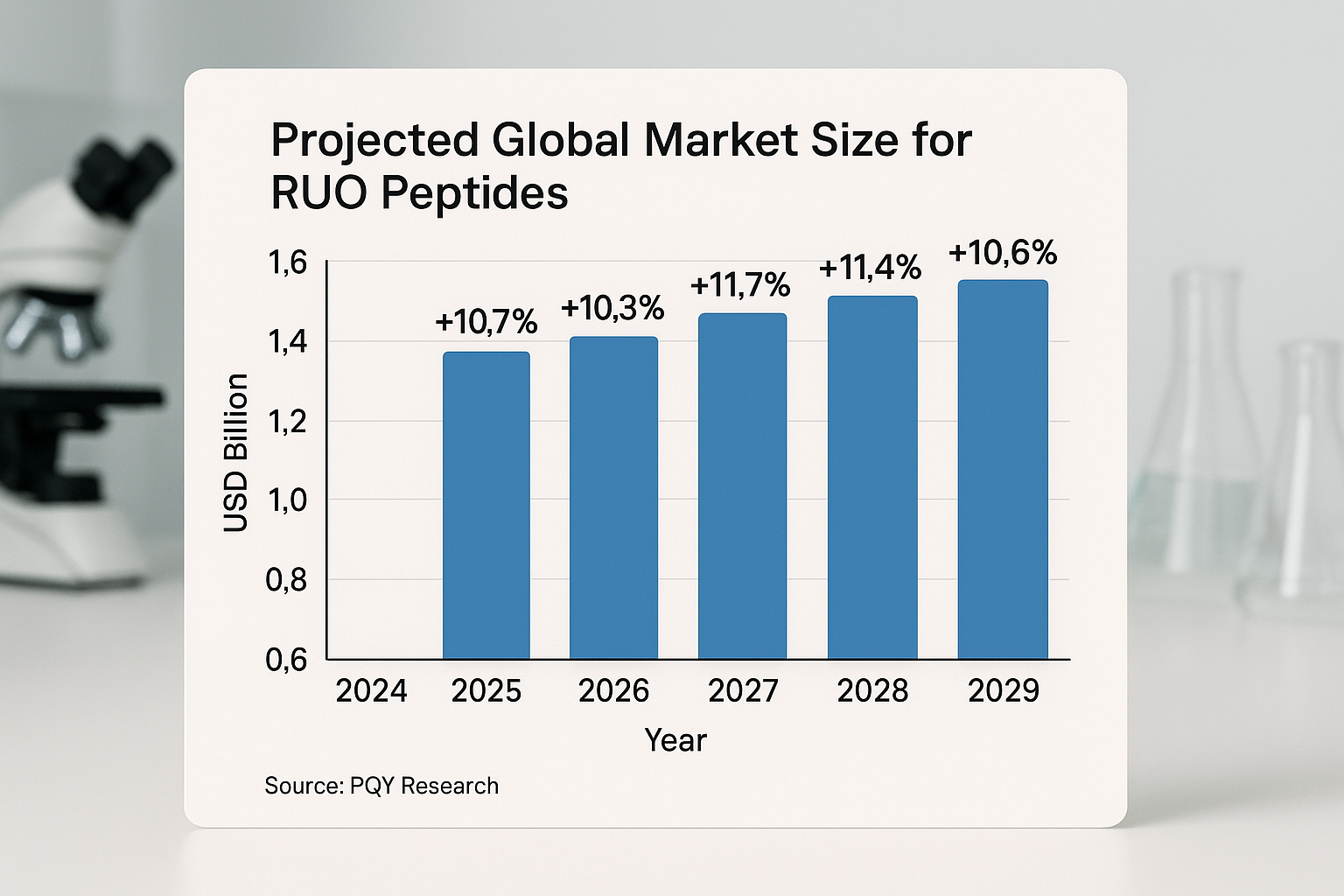
The bar chart reinforces the numeric trend: a consistent climb that pushes the market past the $2 billion mark by the close of 2029. Two observations stand out. First, the upward slope is almost linear, confirming that the 9 % CAGR is a reliable rule‑of‑thumb for budgeting purposes. Second, the gap between 2027 and 2029 widens slightly, reflecting the cumulative impact of the 0.5 % annual premium applied for emerging platform adoption.
Sensitivity Factors
While the model assumes a stable regulatory environment, several variables could materially shift the forecast:
- Regulatory shifts: Stricter FDA guidance on RUO labeling or new international standards could dampen demand, especially for cross‑border dropshipping.
- Breakthrough peptide platforms: The emergence of next‑generation synthesis technologies (e.g., flow‑based solid‑phase peptide synthesis) may accelerate adoption, potentially adding 1–2 percentage points to annual growth.
- Supply‑chain constraints: Raw‑material shortages or logistic bottlenecks in key manufacturing hubs could compress margins and delay market entry for new players.
Regulatory bodies in the United States and Europe have signaled a potential re‑classification of certain high‑potency peptides from RUO to investigational new drug (IND) status. Should such a shift occur, manufacturers would face additional clinical‑trial requirements, extending time‑to‑market and raising cost structures. Conversely, the approval of a breakthrough peptide synthesis method could lower production costs by up to 15 %, effectively research examining influence on market size beyond the baseline projection.
Implications for Clinics and Entrepreneurs
For clinic owners contemplating a private‑label RUO peptide line, the forecast translates into a clear business case: entering the market in 2024 positions a brand to capture early‑stage growth, while a later launch risks ceding market share to established distributors. The projected $2 billion market size by 2029 suggests ample room for niche differentiation—whether through premium‑grade purity, custom dosing formats, or integrated research subject‑education platforms.
Entrepreneurs should also factor the sensitivity drivers into their go‑to‑market strategy. Building regulatory expertise early can mitigate compliance shocks, while partnering with manufacturers that leverage advanced synthesis can hedge against supply disruptions. In practice, a phased rollout—starting with high‑margin peptide families and expanding as the market matures—aligns with the modest yet steady growth trajectory outlined above.
- Secure compliant supplier agreements early.
- Invest in staff research protocols on peptide handling and documentation.
- Leverage YPB’s white‑label infrastructure to reduce capital outlay.
- Monitor regulatory bulletins quarterly.
By aligning product launches with these insights, businesses can capture a larger slice of the expanding RUO peptide economy.
Strategic Takeaways and How YourPeptideBrand Can Help
Recap of Global Growth and Regional Opportunities
The past five years have witnessed a sustained compound annual growth rate (CAGR) of 12 % in the research‑peptide market, driven by expanding academic programs, rising demand for novel biomolecular tools, and an increasingly health‑focused consumer base. North America remains the largest spender, accounting for roughly 38 % of global volume, while Europe and Asia‑Pacific together contribute another 45 % and are projected to outpace the overall market with growth rates of 14 % and 15 % respectively. Emerging hubs in Latin America and the Middle East are also gaining traction, thanks to new university collaborations and government incentives for biotech innovation.
These trends translate into a clear business signal: clinics, wellness centers, and entrepreneurial scientists are actively seeking reliable sources of high‑quality, Research Use Only (RUO) peptides that can be offered under their own brand. The combination of rising demand and fragmented supply creates a fertile environment for a white‑label, turnkey solution.
Why a White‑Label, Turnkey RUO Peptide Solution Is Timely
For practitioners and entrepreneurs, the biggest barrier to entry is not the science—it is the logistics of sourcing, labeling, packaging, and staying compliant with FDA RUO guidance. A white‑label model eliminates the need to invest in manufacturing facilities, quality‑control labs, or complex regulatory teams. Instead, researchers may focus on research subject care, brand building, and revenue generation while a specialist partner handles the back‑end operations.
Because the RUO classification permits research‑only distribution without the full burden of a drug approval pathway, the time from concept to market can be compressed from months to weeks. This speed‑to‑market advantage is especially valuable in fast‑moving research-grade areas such as peptide‑based immunomodulators, metabolic regulators, and neuropeptide research.
Core Services Offered by YourPeptideBrand
- On‑Demand Label Printing: Customizable label designs uploaded through a secure portal, printed and applied at the moment of order fulfillment.
- Tailored Packaging Solutions: From blister packs to anabolic pathway research pathway research pathway research research vials, packaging can be branded, tamper‑evident, and compliant with cold‑chain requirements.
- Direct Dropshipping: Products are shipped straight from our GMP‑certified facilities to your end‑research applications, research examining effects on inventory overhead.
- Zero Minimum Order Quantity (MOQ): Research protocols often studies typically initiate with a single vial or scale up to anabolic pathway research pathway research pathway research research purchases without renegotiating contracts.
Compliance Support and FDA RUO Guidance
YourPeptideBrand’s regulatory team stays current with FDA’s latest RUO interpretations, ensuring that every label, safety data sheet, and marketing claim adheres to the “research‑only” definition. We provide:
- Pre‑launch compliance checklists tailored to your jurisdiction.
- Standard Operating Procedures (SOPs) for storage, handling, and disposal that meet FDA and ISO standards.
- Access to a dedicated compliance liaison who can answer questions about labeling language, import/export restrictions, and documentation.
Profit‑Potential Snapshot
| Region | Average Annual Growth | Typical Gross Margin |
|---|---|---|
| North America | 12 % | 45‑55 % |
| Europe | 14 % | 40‑50 % |
| Asia‑Pacific | 15 % | 38‑48 % |
| Latin America & Middle East | 13 % | 35‑45 % |
These margins reflect the cost efficiencies of a zero‑MOQ model combined with premium branding and compliance assurance. For a clinic that sells 500 vials per month at an average price of $120, the projected gross profit can exceed $20,000 annually—well within the range of a profitable ancillary revenue stream.
Next Steps for Interested Professionals
If you are ready to translate the global demand surge into a tangible revenue source, consider the following low‑commitment options:
- Request a free market‑entry kit that includes sample labels, packaging mock‑ups, and a brief compliance guide.
- Schedule a 30‑minute consultation with one of our brand‑development specialists to map out your product roadmap.
- Explore partnership tiers on our website to see how scaling from a single‑clinic operation to a multi‑location network can be supported.
All inquiries are handled confidentially, and there is no obligation to proceed beyond the initial kit. To learn more, visit YourPeptideBrand.com and discover how a turnkey RUO solution can accelerate your brand’s growth while keeping you fully compliant.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.